Alloy Plating by Heating Stacked Single Layers and the Possibility of its Application in the Future
The 51st William Blum Lecture
This paper was presented at NASF SUR/FIN 2013 in Rosemont, Ill., on June 10, 2013.
By Hideyuki Kanematsu, Suzuka National College of Technology, Japan
Recipient of the 2012 William Blum NASF Scientific Achievement Award
Editor’s Note: This paper was presented at NASF SUR/FIN 2013 in Rosemont, Ill., on June 10, 2013. A printable PDF version is available by clicking HERE.
ABSTRACT
In recent years, the plating industry has had the serious challenge of severe environmental regulations. Various "hazardous" metals, e.g., lead, cadmium, etc., have been the target for regulations one by one. As a result, a pessimistic outlook that any metal could be classified into a regulated one by laws has appeared. However, any element, or materials can present both hazardous and benign situations. In many cases, the so called "hazardous" metals would be toxic, when they exist as an ion, but not as a solid metal. Therefore, alloy plating can be one of the best solutions to avoid disuse by the environmental regulations with minor changes for production. Alloy Plating has been conventionally carried out by coelectrodeposition in solutions. On the other hand, the author has proposed a unique method of alloy coating through the combination of heat treatment and multiple plating. In this lecture, the author shows the results and the possibilities for application in the future.
Introduction
Alloy plating continues to have potential for practical application. Conventionally, it can produce unique properties such as corrosion characteristics, wear resistance, color tone, etc., that cannot otherwise be realized with any single element film. However, recent trends need more innovative developments to solve the practical problems through the utilization of alloy films.
For example, environmental problems seem to hamper the usage of some useful metals (from the functional standpoint at least) at this point. Actually, the use of alloy films can alleviate the environmental burden to some extent. In addition, many emerging needs, such as electromagnetic or optoelectronic properties can appear on the material surface by alloy film formation.
Usually, the alloy film is produced by coelectrodeposition from aqueous solution. However, the author has proposed the combination of surface treatments and heat treatments to produce alloy films in numerous studies.1-25 In this article, I will describe the alloy film formation technique with which my colleagues and I have tried to produce unique materials and describe the characteristics, meaning and the future scope of this technology. Hopefully, this lecture will allow this unique method and technology to produce alloy films and empower many electroplaters and surface finishers to launch new applications of alloy films.
Principles and characteristics of the HSSL process
The proposed alloy film formation process is usually called the Heating Stacked Single Layers Process (HSSL process). As shown in Fig. 1, alloy deposits have been produced to date through coelectrodeposition from aqueous solutions. In this process, the alloying phenomenon occurs during the deposition process. From the standpoint of procedure, the conventional process is composed of a single step.
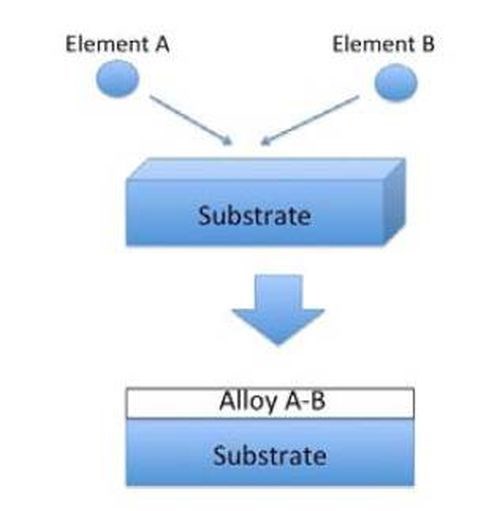
Figure 1 - Schematic illustration for conventional alloy plating.
On the other hand, HSSL process can be divided into two steps. During the first step, the single layers are stacked onto the substrate surface layer by layer. The surface coating method can be chosen freely by the producer in the wide range from plating to cladding or painting. Heat is then added to alloy the stacked layers by mutual interdiffusion on the substrate.
Figure 2 shows the schematic illustration for the HSSL process. Although the process is composed of multiple steps, the process as a whole is not complicated and alloy formation is controlled by the heat treatment process which can be operated more easily than the electrodeposition process.
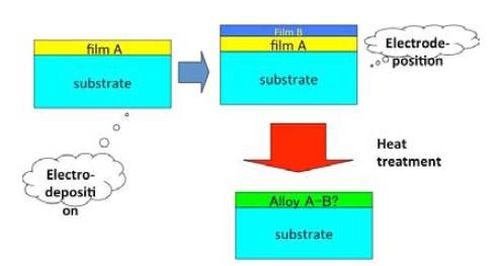
Figure 2 - Schematic illustration for the HSSL process.
Table 1 compares the merits and demerits of conventional alloy plating versus the HSSL process. The most positive characteristic for the HSSL process is its wide selectability in choosing the appropriate method of film formation. This means that one can avoid environmental regulatory issues. Such a state of affairs is desirable for today’s plating industries, which have to struggle with continually emerging environmental regulations. In addition, the multi-step process might not be as complicated, as most plating plants already have thermal treatment facilities to produce stacked single layers. For a number of reasons, many plating processes require other underlayers. From this standpoint, it is highly likely that electroplaters can carry out the HSSL process without significant modification of their facilities.
Table 1 - Advantages and disadvantages of the HSSL process.
| Advantages | Disadvantages |
|---|---|
| Environmental friendliness Easy to operate |
Multistep treatments |
Representative examples of HSSL processes and their applicability
Tin-nickel and tin-zinc alloy plating
Although chromium plating has been effective and highly functional for many purposes, the environmentally harmful hexavalent chromium ion, one of the ionic states for the element, has encouraged the need for substitutes. To that end, a number of substitutes have already reached the market, including tin-nickel and tin-cobalt plating, among others. Regarding the former, the author has investigated the alloying phenomenon in detail. Figure 3 shows a schematic illustration of the process.
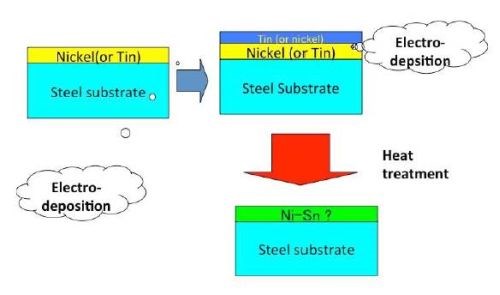
Figure 3 - Schematic explanation of an HSSL process used to produce a Ni-Sn alloy film.
There are two ways to produce stacked single layers of tin and nickel. The first is to produce a tin layer followed by a nickel layer and the second is to produce a nickel layer followed by a tin layer. When a fluoboric acid bath was used for tin and a Watts bath was used for nickel, tin could be dissolved during the subsequent electrodeposition of nickel from the Watts bath. As a result, the appropriate tin and nickel stacked layers could not form. If those inappropriately stacked layers had been heat treated, the intermetallic compound layers would not have been produced. Therefore, nickel was deposited first and then tin could be deposited on the nickel.
Since the melting point of tin layer is about 230°C, the subsequent heat treatment was carried out just above or below the melting point of tin. In that temperature range, the tin layer, the lower melting point metal phase, was melted, while the nickel layer, the higher melting point metal phase, remained in the solid state. Since the melting point of nickel is much higher (1455°C) and would require relatively elaborate furnaces for the heat treatment, the relatively low temperature heat treatment would be desirable from a practical standpoint. In such a case, there are two possibilities - the heat treatment temperature held above the melting point of tin or below it. In the former case, the tin layer would be melted, while nickel layer would be solid. In the latter case, both layers would be solid. The two cases have their own different mechanisms to form an alloy film. In both cases, the mutual diffusion between the two stacked phases on the material surface is the key mechanism. However, the solid-liquid interaction would occur in the former, while the solid-solid one would occur in the latter. In the former, the diffusion rate would be very high, since the solid nickel would diffuse very easily into the liquid tin phase or vice versa. As a result, the former case would take several minutes for alloy formation to result, while the latter case would take several days or weeks. In any case, some intermetallic compound layers of tin and nickel would form through mutual diffusion.
One may ask if the liquid tin phase would be lost during the heat treatment process. However, we confirmed that such a phenomenon never occurred, meaning that the mutual diffusion that formed the intermetallic compound layers occurred at very high rate. Figure 4 shows the schematic illustration for the alloy film formation in that case.
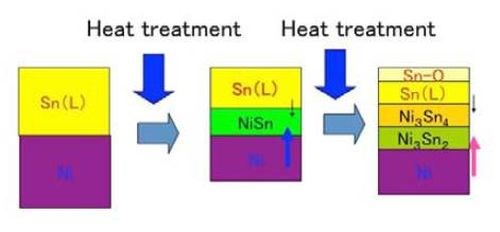
Figure 4 - Schematic illustration for the formation of intermetallic compounds between tin and nickel.
The film of intermetallic compounds produced between the tin and nickel layers has high corrosion resistance and relatively high hardness. The polarization curve of the alloy film specimen measured in 3% NaCl solution showed that no corrosive dissolution occurred in the usual potential range for a steel substrate and that it had very high corrosion resistance. On the other hand, Fig. 5 shows the Vickers hardness on the surface of specimens measured by a Micro Vickers hardness testing machine. The hardness depended on the heat treatment temperature. When the specimen was heated at 550°C, the maximum hardness was obtained at the vicinity of surface of the specimen (750VHN). The color tone also depended on the heat treatment temperature and usually it generally was a dark gold or yellow ochre. It was noteworthy that the color tone was different from that obtained by the conventional alloy electrodeposition process. In that case, it was pink tinted.
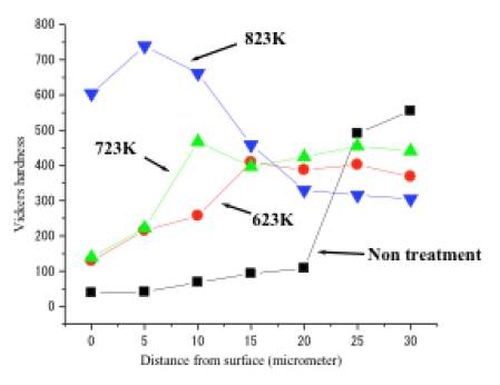
Figure 5 - Vickers hardness for a tin-nickel alloy film produced by the HSSL process.
A tin-zinc alloy film could be formed in the same way. The alloy film could be applied as a substitute for cadmium plating, which has been used for aircraft parts. During World War II, cadmium was in short supply for a number of reasons. Around that time, tin-zinc alloys were used as a substitute. However, today cadmium substitutes are considered from an environmental standpoint. The phase diagram of the tin-zinc system clearly shows a eutectic. To produce high corrosion resistance films, a zinc-rich alloy film would be desirable (more than 90% zinc, conventionally). Also in this system, the order of stacked single layers are arbitrary (zinc as an underlayer and tin as an overlayer, and vice versa). As a concrete example, we produced stacked single layers composed of tin films from an acid tetrafluoboric tin bath and zinc films from a zinc sulfate bath and heat-treated them at 350-400°C. X-ray diffraction analysis showed that the alloy phases were mixed crystals of zinc and tin. For this process, versatile combinations of heat treatments and plating processes would be possible. For example, the author used a laser treatment process with some plating methods to shorten the process time.
Antibacterial alloy films
Using the principles for the combination of alternating plating for the formation of single layers and the subsequent heat treatment, many alloy films are possible to produce. My colleagues and I have investigated many elements and combinations thereof. However, it is essential that the application be considered from the practical viewpoint. For the studies of the HSSL process, we began with tin alloy films, as described above.
Tin plating has been used in the food processing industry. Considering this, we found a second focus on the antibacterial effects and worked to produce an antibacterial effect in tin plating by adding other antibacterial elements through the HSSL process. Generally speaking, tin shows little antibacterial effects. Table 2 shows the test results of the antibacterial effect for tin plated steels.
The test method, commonly called the Film Covering Method (ISO 22196), provides useful information needed for practical applications. The specimens are put in a plastic Petri dish, while the bacterial solution is prepared as follows. The bacteria are incubated in 10 mL of a nutrient broth for 24 hr at 35°C, then diluted two-thousand fold with sterilized water and established as a bacterial solution. The diluted bacterial solution is applied to the specimen (16 μL/cm) and a polymer film was then laid over the solution. The sample was kept in an incubator for 24 hr at 35°C.
After the incubation, a solution of 10 mL of sterilized water containing 200 μL of Tween 80 (a nonionic surfactant and emulsifier) was introduced into the plastic Petri dish and the bacteria attached to the specimen and polyethylene film were washed into the aqueous solution. To determine the number of viable cells, serial decimal dilutions of the cell suspension were made, a 0.1 mL portion of which was uniformly spread on an agar medium. The plate was incubated at 35°C for 24 hr and the colonies formed were counted. The viable cell count was represented as colony forming unit per milliliter (CFU/mL). The final colony formation number was measured to evaluate the antibacterial properties.27 As shown in Table 2, the number of bacteria was not reduced after 24 hr had passed. Rather, it increased slightly. At minimum, a decrease of bacterial numbers on the order of 10 to the second power would be required to confirm the antibacterial effect.
Table 2 - Effect of the presence of tin-plated steel on bacterial count.26
| Time (Hrs) |
Specimens |
E-coli |
|---|---|---|
| 0 | Control | 9.5 × 104/plate |
| 24 | Control | 2.04 × 106/plate |
| 24 | Tin-plated steel | 7.51 × 106/plate |
Figure 6 shows a schematic illustration for making Sn-Cu alloy films. In the example, the copper film was prepared by electroplating. The tin film was produced by a magnetron sputtering process. However, any combination (e.g., even if all films were produced by electroplating) would lead to the same result. The heat treatment temperature was set to 300°C, just above the melting temperature of tin.
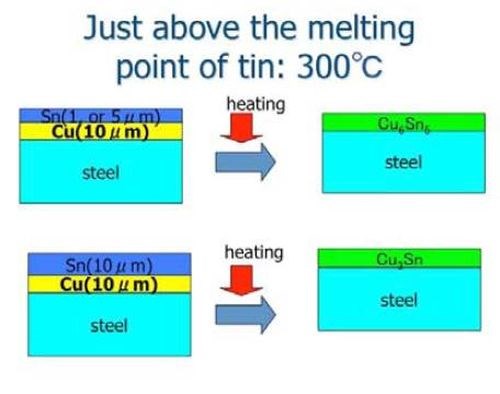
Figure 6 - Schematic illustration for producing a tin-copper alloy film by HSSL.26
The tin layer was melted after the specimens were heated to the temperature. The mutual diffusion rate then increased significantly to form intermetallic compounds of tin and copper. When the film thickness of the tin layer was relatively small (5 mm tin and 10 mm copper), the main intermetallic compound layer was Cu6Sn5, while the thicker film specimen contained Cu3Sn. Table 3 shows the antibacterial effects for various tin-copper plated films by the film covering method. As Table 3 shows, tin-copper alloy films clearly showed strong antibacterial effects.
Table 3 - Antibacterial effects of tin-copper plated films by the Film Covering Method.26
| Time (Hrs) |
Specimens | Escherichia coli | Klebsiella pneumonia ST101 |
Staphylococcus aureus 209P |
| 0 | Control | 9.5 × 104/plate | 1.71 × 105/plate | 1.09 × 105/plate |
| 24 | Control | 2.04 × 106/plate | 6.76 × 106/plate | 8.3 × 105/plate |
| 24 | Sn-Cu Plating | 0 | 0 | 0 |
My colleagues and I confirmed that the antibacterial effect of intermetallic compounds for tin-based films was also observed by adding silver17,28-30 and palladium31,32 to tin films. However, from a practical viewpoint, silver seems to be the most important in addition to copper. Actually, most of the patents in Japan have been concentrated on silver and copper compounds, while titanium oxides were another option due to their photocatalytic effect. Many other elements may work equally well. This might be a good future research topic, since surface finishing for antibacterial effects will be very important for aging societies in advanced countries.
Applicability and limitations
When one wants to use HSSL process, are any alloy systems available, so long as the stacked single layers can be formed? The answer is obviously negative. However, we have to know in advance how we could tell the possible cases from impossible ones.
One of the ways to wean out the practical possibilities lies in the use of phase diagrams. For example, the nickel-tin phase diagram clearly shows the intermetallic compound phases, as shown in Fig. 7. On the other hand, the silver-nickel system would not lead to the formation of alloy films, based on the phase diagram for the two elements shown in Fig. 8. When the phase diagram shows some intermetallic compounds, alloying is possible. Therefore, the phase diagram is a guideline for the HSSL process.
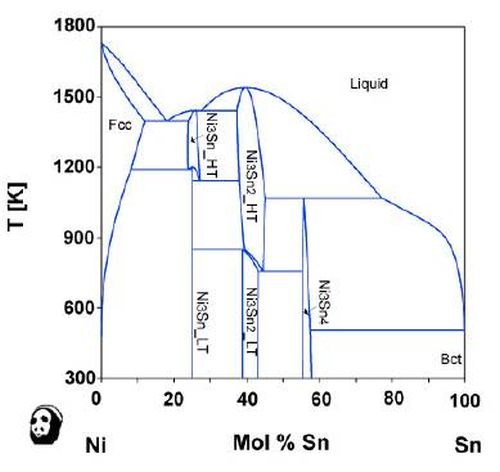
Figure 7 - Phase diagram of nickel and tin (Provided by the National Institute for Materials Science (NIMS)).
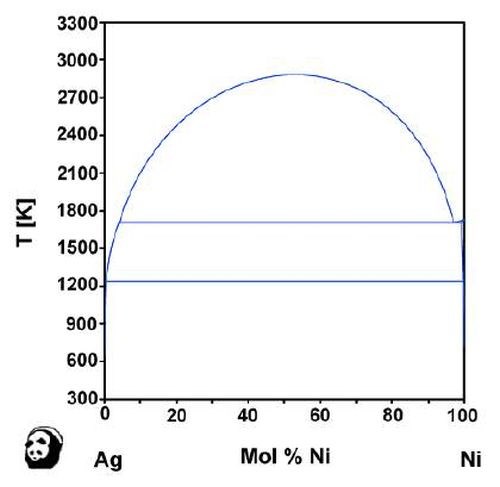
Figure 8 - Phase diagram of nickel and silver (Provided by the National Institute for Materials Science (NIMS)).
On the other hand, the simulation software that Dr. Yoshitake at NIMS developed on the NIMS website may work well to predict the effectiveness of alloying for practical purposes.33 The software was originally developed to predict the experimental results in advance, i.e., whether a substrate element A were to diffuse into the film element B to the top of the film or not.34-36 Figure 9 shows the possible cases for the calculation. The software could well solve the problems from the practical viewpoint. For example, Fig. 10 shows the case for the stacked single layers of nickel and silver. In this case, nickel exists as the upper layer and silver is the lower one. Vacuum or inert gas was chosen as the atmosphere. The figure shows the screen shot for the virtual investigation and calculation and suggests that silver under nickel could diffuse into the upper nickel layer and appear on top of it on the top surface by appropriate heating. We have not yet investigated this case under actual conditions. However, the stacked single layers and heating might make it possible to impart an antibacterial effect to the surface of nickel plating in the future.37
Figure 9 - Decision branches for segregation simulation on the web page of Surfseg provided by the National Institute of Materials Science (NIMS); http://surfseg.nims.go.jp/SurfSeg/select.html.
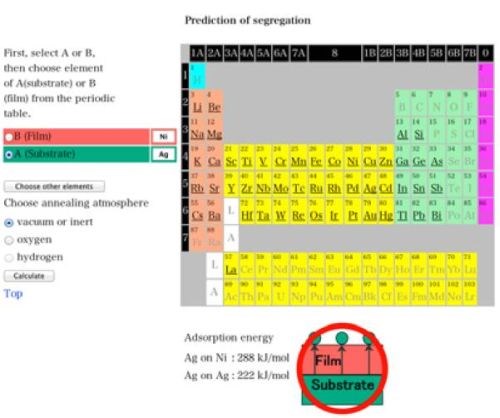
Figure 10 - A calculation result for a nickel-silver alloy film by heating by Surfseg (NIMS).
Future scope – from antibacterial effects to biofilm control
Bacteria and fouling caused by them pose significant concerns in the food processing, sanitary and medical industries. However, we human beings know that bacteria do not exist alone, but rather they live in colonies in biofilms on materials.
A biofilm can be defined as a structured community of bacterial cells enclosed in a self-produced polymeric matrix and adherent to an inert or living surface.38 Figure 11 shows the mechanism of biofilm formation. Bacteria which flow in oligotrophic environments, e.g., air, various aqueous solutions like sea, river, clean water, etc., are generally called planktonic bacteria. They are always seeking nutrients to survive. At the same time, they need to hide themselves from their natural predators and shelter from the flowing media in which they find themselves, which may sweep them away. For those reasons of survival, bacteria attach themselves to material surfaces where organic compounds are absorbed as nutrients. The bacteria thus migrate to the nutrient-enriched surface and attach there.
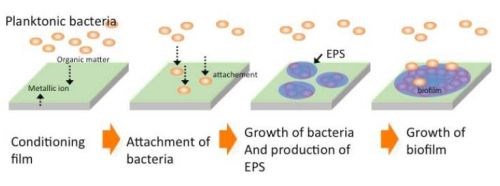
Figure 11 - Schematic illustration of biofilm formation.39
For a while, they repeat the attachment and detachment process, and finally the number of attached bacteria becomes to dominate the number of detached ones at a certain time. This process goes on, with bacteria repeatedly attaching and detaching themselves, but ultimately increasing in number attached to the surface. When the number of bacteria attached to the surface exceeds a threshold value, they simultaneously secrete polysaccharides and surround themselves with it. At this point, a biofilm forms and it grows, increasing the number of bacteria, polysaccharides, DNA, etc. (referred to as exopolymeric substances, or EPS) and incorporating various elements such as silicon, calcium, magnesium, zinc, etc.39
Such biofilms can affect many industrial problems such as corrosion, sanitary/food processing industry, infection of biomaterials, shipment problems, etc., as shown in Fig. 12. Of particular interest to this laboratory is the problem of fogging of glasses by water staining and other processes, which can lead to a serious setback for the application of glasses to heliostats.
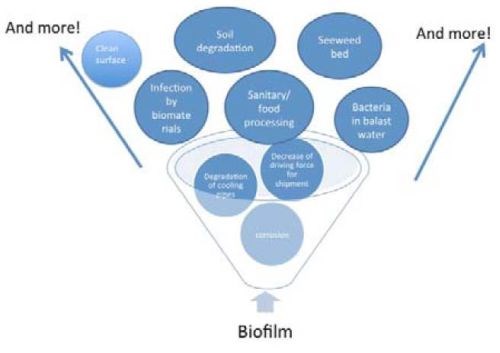
Figure 12 - Industrial problems caused by biofilms.40
The heliostat is one of a number of apparatuses related to photovoltaic power generation. It reflects the sunlight in the polar direction with a flat mirror. The polar axis supporting the flat mirror makes a 360° roll each day, chasing the sunlight and reflecting it to the measuring device. Its efficiency depends on the mechanical accuracy of the fine driving apparatus. However, the decreasing reflectivity of glass being fogged causes serious deterioration of the power generation efficiency. The fogging of glass itself is never caused by biofilms, but rather simple water stains. However, once the biofilm forms on a glass surface, the sticky EPS produced by bacterial activities can firmly fix the stain and fouling on the glass surfaces and the performance and transparency significantly decrease as a result.41,42
As countermeasures, certain special surface treatments such as the application of a water-shedding coating have been already devised. However, those have been insufficient to solve the problem completely. Antibacterial coatings could well offer an improved solution. The HSSL process may be applied to solving the problem in the future. As shown in Fig. 13, such a novel countermeasure may also address the fogging problem on touch panel sensors, whose market will be very big in the near future.
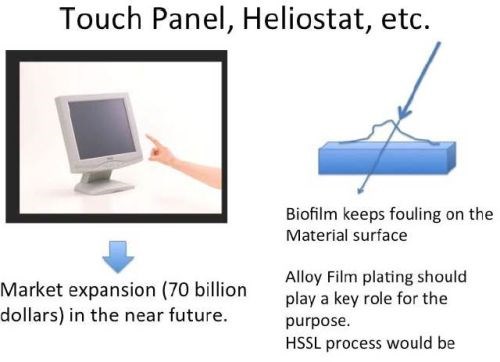
Figure 13 - The applicability of the HSSL process and anti-bacterial coatings to biofilm control.
Acknowledgment
Part of this project was supported financially by NEDO (New Energy and Industrial Technology Development Organization) and was entitled “The Development of Minimum Life Cycle Cost Heliostat by the Utilization of Biotechnology and Anti-Fogged Glass.” I also gratefully thank Dr. Michiko Yoshitake of the National Institute of Materials Science for our collaborative project between NIMS and SNCT over many years. I also appreciate the great society and the people of the IMF (Institute of Materials Finishing) in the United Kingdom who have given me much inspiration and friendship. The former Presidents in Suzuka National College of Technology, as well as Prof. Yasutsugu Nitta, the current President, who have always encouraged me to participate in the NASF/AESF SUR/FIN, although I had many college duties. I very much appreciate their generous and kind consideration. Finally, I highly appreciate all of my colleagues in my research activities and the NASF (AESF) members, particularly Mr. Bob Srinivasan, Dr. James Lindsay and Mr. Tony Revier, for their long support and friendship over many years. Without their support and encouragement, my contributions to the NASF might have not continued for so long.
Full references
1. T. Oki, R. Ichino, H. Kanematsu, T. Inoue & Y. Kunieda, “Separation & Recovery of Al & Zn from Galvanized Steels, Using Various Molten Metals,”Proc. AESF SUR/FIN 1998, NASF, Washington, DC (1998); pp. 718-724.
2. H. Kanematsu, T. Kobayashi & T. Oki, “Thermal Alloying of Tin/Nickel Films as Substitutes for Chromium Plating, Proc. AESF SUR/FIN 2000, NASF, Washington, DC (2000); pp. 75-80.
3. H. Kanematsu, T. Kobayashi, N. Wada & T. Oki, “Alloying Behavior of Tin-Zinc from Stacked Single Layers,” Proc. AESF SUR/FIN 2002, NASF, Washington, DC (2002); pp. 632-638.
4. H. Kanematsu, T. Kobayashi, N. Wada & T. Oki, “Tin-Nickel Alloy Films Produced from Stacked Single Layers by Heating and Their Characteristics,” Proc. AESF SUR/FIN 2002, NASF, Washington, DC (2002); pp. 685-694.
5. H. Kanematsu, N. Wada, N. Takayama, T. Ogura & Takeo Oki, “Titanium Oxide Powder Coating for High Emissivity,” Proc. AESF SUR/FIN 2002, NASF, Washington, DC (2002); pp. 830-836.
6. H. Kanematsu, N. Hirai, T. Tanaka & T. Oki, “Topographical Observation of Tin Precipitation for Pulse Plating by Using SEM and AFM,” Proc. AESF SUR/FIN 2003, NASF, Washington, DC (2003); pp. 212-219.
7. H. Kanematsu, T. Kobayashi, N. Wada & T. Oki, “Corrosion Characteristics of Alloy Films Produced by HSSL Process,” Proc. AESF SUR/FIN 2003, NASF, Washington, DC (2003); pp. 673-682.
8. N. Hirai, H. Kanematsu, T. Tanaka & T. Oki, “In Situ Electrochemical AFM Observation for Dissolution of Plated Copper in Aqueous Solution,” Proc. AESF SUR/FIN 2004 & Interfinish 2004 World Congress, NASF, Washington, DC (2004); pp. 835-839.
9. H. Ikigai, H. Kanematsu, Y. Kikuchi & T. Oki, “Various Plating Metals and their Antimicrobial Effect,” Proc. AESF SUR/FIN 2004 & Interfinish 2004 World Congress, NASF, Washington, DC (2004); pp. 996-1005.
10. H. Kanematsu, S. Fujimoto, K. Nogi & T. Oki, “Application of Inorganic Silicon Sealer to Anodic Oxide Coating,” Proc. AESF SUR/FIN 2004 & Interfinish 2004 World Congress, NASF, Washington, DC (2004); pp. 478-485.
11. H. Ikigai, H. Kanematsu, K. Kuroda & A. Ohmori, “Antibacterial Activity by Alloying of Tin and Copper Plating,” Proc. SFIC SUR/FIN 2005, NASF, Washington, DC (2005); pp. 497-503.
12. H. Kanematsu, H. Ezaki, N. Yanagi & T. Oki, “Alloy Film Production of Tin-Copper Through Heat Treatment of Stacked Layers,” Proc. SFIC SUR/FIN 2005, NASF, Washington, DC (2004); pp. 489-496.
13. H. Kanematsu, H. Ikigai, K. Kuroda & A. Ohmori, “Antifungal Characteristics of Some Metal Plating,” Proc. SFIC SUR/FIN 2005, NASF, Washington, DC (2005); pp. 489-496.
14. H. Kanematsu, H. Ikigai & M. Yoshitake, “Various Properties of Tin Copper Alloy Filim Produced by Heat Treatment,” Proc. SFIC SUR/FIN 2006, NASF, Washington, DC (2006); p p. 864-870.
15. H. Kanematsu, H. Ikigai & M. Yoshitake, “Alloying of Stacked Nickel/Zinc Films Through Heat Treatment,” Proc. SFIC SUR/FIN 2006, NASF, Washington, DC (2006); pp.360-369; pp.360-369; also in J. Applied Surface Finishing, 2 (1), 43-46.
16. H. Kanematsu & K. Nio, “Quantification of Human Sensitivity Affected By Surface Color Tone of Plating,” Proc. SFIC SUR/FIN 2006, NASF, Washington, DC (2006); pp. 360-369.
17. H. Kanematsu, H. Ikigai & M. Yoshitake, “Alloying of Tin Plating film with Silver by Combination of Multistage Plating and Heat Treatment for Antibacterial Property,” Proc. SFIC SUR/FIN 2006, NASF, Washington, DC (2006); pp. 13-15; also in J. Applied Surface Finishing, 3 (3), 114-118.
18. H. Kanematsu, A. Nakao & M. Yoshitake, “Non-reactive Stacked Surface Layers and Their Heat Treatment Behavior - The Application to Nickel Plating,” Proc. NASF SUR/FIN 2007, NASF, Washington, DC (2007); pp. 253-261: also in J. Applied Surface Finishing, 2 (4), 308-311.
19. H. Kanematsu, D.M. Barry & J. Benham, “Quantifying Human Impressions of Glossy Plated Surfaces Using the Semantic Differential Technique,” Proc. NASF SUR/FIN 2007, NASF, Washington, DC (2007); pp. 501-509.
20. H. Kanematsu, D.M. Barry & J. Benham, “Analyses and Discussion of Human Sensation/Reaction to Glossy Surfaces,” Proc. NASF SUR/FIN 2008, NASF, Washington, DC (2008); p. 206-218.
21. H. Kanematsu, K. Murata, A. Ogawa & Y. Miyano, “Sn-Zn Alloy Films Produced by HSSL and Their Environmental Characteristics,” Proc. NASF SUR/FIN 2008, NASF, Washington, DC (2008); p. 309-321.
22. H. Kanematsu, H. Ikigai, S.A. Campbell & I.B. Beech, “Sn-Ag Alloy Plating Films Mitigating Biofilm Formation,” Proc. NASF SUR/FIN 2009, NASF, Washington, DC (2009).
23. H. Kanematsu, K. Murakami & K. Nakata, “Surface Finishing of Concrete Structure by a Silane Series Solvent,” Proc. NASF SUR/FIN 2009, NASF, Washington, DC (2009).
24. H. Kanematsu, K. Shirakihara & D. Kuroda, “Change of Residual Stress with Alloy Film Formation by HSSL Process,” Proc. NASF SUR/FIN 2009, NASF, Washington, DC (2009).
25. A. Ogawa, N. Okuda & H. Kanematsu, “Comparison of Biological Toxicity of Several Plating Products by Mammalian Cells,” Proc. NASF SUR/FIN 2009, NASF, Washington, DC (2009).
26. S. Suzuki & H. Kanematsu, “Anti-Microbial Treated Metals for Commercial Use, in Report on Effect of Biofilm and Microbes on Materials. Division of Microstructure and Properties of Materials, Iron & Steel Institute of Japan, Y. Sato, Ed., 2011, Iron and Steel Institute of Japan, Tokyo; pp. 77-82.
27. H. Kanematsu, H. Ikigai & M. Yoshitake, “Evaluation of Various Metallic Coatings on Steel to Mitigate Biofilm Formation,” International Journal of Molecular Science, 10 (2), 559-571 (2009).
28. D. Kuroda, H. Kanematsu, H. Ikigai, M. Yoshitake & S. Yagyu, “Formation of Antibacterial Tin-Silver Films Through Heat-Treatment Alloying Process,” CAMP-ISIJ, 20, 1185 (2007).
29. H. Kanematsu, H. Ikigai & M. Yoshitake, “Antibacterial Tin-Silver Plating by the Combination of Multistage Plating and Heat Treatment, Journal of Applied Surface Finishing, 3 (3), 114-118 (2008).
30. H. Kanematsu, A. Nakao & M. Yoshitake, “The Thermal Diffusion Behavior of Non-Reactive Silver with Nickel on Steel Substrates: The Application to Nickel Plating,” Journal of Applied Surface Finishing, 2 (4), 308-311 (2007).
31. H. Kanematsu, H. Ikigai, D. Kuroda, M. Yoshitake, S. Yagyu & A. Ogawa, “Tin-Palladium Alloy Formation by Heat Treatment-Alloy Plating Process and Antibacterial Effect of its Film, Netsushori, Journal of the Japan Society for Heat Treatment, 49 (5), 274-278 (2009).
32. H. Kanematsu, K. Nio, D. Kuroda, H. Ikigai, M. Yoshitake & S. Yagyu, “Formation of Tin-Palladium Films by Heat-Treatment Alloying Process and Its Surface Properties,” CAMP-ISIJ, 20 (6) 1186 (2007).
33. Surfseg provided by National Institute of Materials Science; http://surfseg.nims.go.jp
34. M. Yoshitake, Y-R. Aparna & K. Yoshihara, “General Rule for Predicting Surface Segregation of Substrate Metal on Film Surface,” J. Vacuum Science and Technology A, 19, 1432-1437 (2001).
35. M. Yoshitake, Y-R. Aparna & K. Yoshihara, “Novel Interface Modification Technique Applied from the Top of a Coated Layer. “J. Vacuum Science and Technology A, 19, 2612-2616 (2001).
36. M. Yoshitake, Y-R. Aparna & K.Yoshihara, “Modification of Coating Layer/Base Material Interface,” J. Vacuum Society of Japan, 44 (3), 151-154 (2001).
37. H. Kanematsu, M. Yoshitake & D. Kuroda, “Anti-bacterial Alloy Film Formation by Heat Treatment and The Design Possibility Based on Segregation Prediction System, Netsushori, J. Japan Society for Heat Treatment, 52 (3), 143-149 (2012).
38. J.W. Costerton, P.S. Stewart & E.P. Greenberg, “Bacterial Biofilms: A Common Cause of Persistent Infections, Science, 284 (5418), 1318-1322 (1999).
39. H. Kanematsu, T. Kougo, D. Kuroda, H. Itoh & R. Noorani, “Prevention Coating Against Biofilm Formation,” Proc. SUR/FIN Asia-Pacific 2012, S. Hemsley, E., Singapore Surface Engineering Association, Singapore (2012); pp. 52-56.
40. H. Ikigai & H. Kanematsu, “Mechanism for Antibacterial Effects by Metallic Elements and their Inhibition of Bacterial Infections,” J. Japanese Society for Biomaterials, 29 (4), 232-239 (2011).
41. H. Kanematsu, T. Kogo, H. Itoh, N. Wada & M. Yoshitake, “Fogged Glass by Biofilm Formation and its Evaluation, Proc. MS&T 2013, ASM International, Materials Park, Ohio (2013).
Related Content
An Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreProducts Finishing Reveals 2024 Qualifying Top Shops
PF reveals the qualifying shops in its annual Top Shops Benchmarking Survey — a program designed to offer shops insights into their overall performance in the industry.
Read MoreA Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.
Read MoreLiquid Chrome Vs. Chromic Acid Flake
Contemplating how to continue offering chromic acid services in an increasingly stringent regulatory world? Liquid chrome products may be the solution you’re looking for.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreMasking Solutions for Medical Applications
According to Custom Fabricating and Supplies, a cleanroom is ideal for converting, die cutting, laminating, slitting, packaging and assembly of medical-grade products.
Read More





























