Are Ionic Liquids the Future of the Surface Treatment?
This paper is focused on the application of ionic liquids related to surface modification and metal deposition.
by Patrick Benaben, Ecole Nationale Supérieure des Mines de Saint-Étienne
ABSTRACT
This paper is focused on the application of ionic liquids related to surface modification and metal deposition. A description of ionic liquids (definition, structural aspects, preparation and properties) explains the physical and chemical characteristics of the liquids and the potential application to general chemistry as a "green chemistry" product. The paper is more dedicated to surface treatment and the improvement that we can expect from the substitution of water solutions by ionic liquids. Development of this technology towards what seems the most three promising processes will be discussed:
1. Metal polishing, including titanium and steel,
2. Hard chromium deposition, as a potential substitute for aqueous chromium plating,
3. Aluminum deposition, for new decorative and anti-corrosion applications.
Results obtained in our research activities and from more general experiments published in the literature will be discussed, giving an overview of what is currently in development and what kind of applications are feasible. The paper concludes with a general discussion of future potential industrial developments, from a scientific and economic point-of-view.
Keywords: ionic liquids, non-aqueous processes, metal polishing, hard chromium substitutes, aluminum deposition
Introduction
Liquefaction of a solid or a mixture composed of solids in the form of mineral and/or organic compounds can be accomplished by supplying energy to the mixture after its preparation. In this study, this is done by increasing temperature until the interatomic bonds between the atoms of the crystals are broken.
Ionic liquids are defined as:
- Salts which are intrinsically liquids at the temperature of use,
- Liquids obtained by mixing salts (organic and/or mineral) at a temperature below 200°C (392°F) by formation of a deep eutectic liquid complex.
For purposes of discussion in this paper however, the ionic liquids discussed are those which are liquids at a temperature below 100°C (212°F).
The liquefaction process is a function of the asymmetry between cation and anion size, and this asymmetry induces a distortion of the link between the two species, allowing separation and thus liquefaction of the product. In general, it is the cationic species which are larger.
General Considerations
At the beginning of the 20th century, the first low temperature ionic liquid to be discovered was ethyl ammonium nitrate (C2H5NH3+ - NO3-, but the real significance of ionic liquids became apparent with the development of compounds produced from such cations as imidazolium and pyridinium compounds as alkyl-pyridinium chloride and alkyl-arylimidazolium in the last 15 years. The development of these ionic liquids is linked to their use in combination with aluminum chloride for deposition of aluminum and aluminum alloys.
For anionic species, those most used are the halide ions, such as chloride, fluoride, iodide and bromide. Others include hexafluorophosphates (PF6-) and tetrafluoroborates (BF4-), and the more complex anions trifluoroacetate (CF3CO2-) and triflate (CF3SO3-).
More recently, the compound 2-hydroxy-N,N,N-trimethylethanaminium (choline ion) has been used in numerous applications, in particular in electrolytic applications for new processes, such as those in the European research program "IONMET."
Given the preceding discussion, it is possible to create a multitude of new compounds and consequently ionic liquids. Currently, more than 100,000 new compounds have been synthesized. Nonetheless, such a multiplication and abundance of products developed for future application poses some difficulties, related to the REACH (Registration, Evaluation, and Authorization of Chemicals) regulations.
Finally, as a positive and very interesting result, it is possible to adapt the formulation of the compounds to the desired physical and/or chemical characteristics.
From this general description, the most important characteristics of ionic liquids can be summarized as follows:
- A system with a large size difference between cation and anion produces a very asymmetrical structure which is characterized by a low liquefaction temperature at ambient pressure.
- The nature of the anion is also very important in defining the general properties of the compound.
- The temperature of liquefaction can be adjusted by the proper selection of the ions.
Other characteristics include:
- Lower conductivity in ionic liquids, compared to classical aqueous salt solutions
- Non-flammable liquids with a very low vaporization pressure at temperatures below 150°C (302°F)
- Large liquid temperature range
- High thermal stability (up to 250-300°C; 482-572°F)
Given these properties and characteristics, ionic liquids have found use in such chemical applications as catalysts and biocatalysts, chemical polymerization, materials (metals) preparation, electrochemistry, organic chemistry (for synthesis of new materials) and product recycling and surface preparation (etching).
Ionic liquid solvents are relatively new. Due to the nature of the solution and the active ions contained therein, the physico-chemical reactions are very different from those resulting with the same ions in aqueous solution. As a consequence, novel products and reactions are able to take place using ionic liquids. The differing thermodynamic behavior in chemical or electrochemical reactions can lead to very innovative concepts.
In particular, they are considered to have a very important contribution in the area of "green chemistry" development, given, for example, their low flammability and evaporation behavior. Nonetheless, it should be noted that ionic liquids are not intrinsically "green." Determining the nature of the toxicity of cations and anions, if any, is still important in evaluating their effects on health and the environment.
Application to Surface Treatment and Plating
The use of ionic liquids in the field of surface treatment and electroplating is increasing. Until now, these operations have primarily used aqueous solutions. There are exceptions, in particular, the electrolytic deposition of aluminum metal using organic solvents.
The physico-chemical properties of ionic liquids are completely dependent on the nature and the size of constituent ions. For instance, ionic liquids formed with chloride, bromide or trifluoracetate ions are highly soluble in aqueous media. Inversely, for anions like hexafluorophosphate or bis(triflyl)amide, solubility in water is limited and in general, the solution presents two immiscible phases.
These new properties, also issuing in part from a larger electrochemical window than aqueous solutions, allow the development of new chemistries and electrochemistries for surface treatment and plating conditions with an important modification of the general rules imposed by the use of water solvents. Therefore, the electrochemistry defined by Pourbaix diagrams (electrochemical potential vs. pH) is fundamentally changed.
Using these new solvents brings important modifications of the general rules of plating and surface treatment. Classical reference electrodes, chemical parameters such as pH equivalent, and physical characteristics such as conductivity and viscosity (with their influence on diffusion and migration of active species) are redefined and have to be studied in detail to use ionic liquids properly.
Given our current knowledge of ionic liquids, what follows is a discussion of what kind of applications are foreseeable for future plating and surface treatment technologies. Based on our experience in the IONMET European Research Program (New Ionic Liquid Solvent Technology to Transform Metal Finishing Products and Processes), a large program (30 partners in the frame program 6th PCRD) now completed, electropolishing (surface treatment) and some applications in electroplating seem for us to be the more interesting paths for the future development of ionic liquids.
Electropolishing
Electropolishing is accomplished by electrolytic (anodic) dissolution, the opposite of the metal electroplating process. In general, electropolishing is used because it allows one to produce better surface aspects than mechanical polishing and also because in general there is no modification of the surface structure (no modification of crystallization). In some cases, the polished surface exhibits increased corrosion and wear resistance.
Generally speaking, industrial processes use aqueous sulfuric and phosphoric acid solutions in an anodic process with an electrical efficiency of about 20%, due to oxygen release and evolution.
Aqueous processes developed industrially are currently efficient and robust, but they produce large volumes of sludge due to the formation of compounds, including metals associated with the anodic dissolution (Fe, Cr, Ni, Zn, etc.). The performances of the used solutions decreases as a function of the concentration in sludge of the solution. Thus, it is necessary to treat and purify the solution to maintain the efficiency of the process at a high level.
In this kind of application, different compositions of ionic liquids have been studied to increase the performance of the solution used. Ionic liquids formed with glycol seem to present the best results, in particular, a proprietary solution of ethylene glycol.** However, this product is reputed to be carcinogenic and studies are in progress to substitute this product with other glycol compounds.
Regarding the process itself, it seems that there is no viscous film formed at the surface of the metal (anodic part). Comparing the I = f(V) curves for the two processes (ethylene glycol and acidic aqueous solutions) shows that the peak corresponding to the dissolution by acidic solution (i.e., the formation of a viscous film) is not present in the curve for the ionic liquid. It appears that with ionic liquids, the efficiency of the electropolishing is approximately constant even if the solution is polluted by metal or impurities from the process itself. It is also interesting to note that after saturation of the solution (reaching the solubility limit, 10% for iron) precipitation of metals occurs. It is thus possible to extract (by filtration for instance) the excess of metal and so have a solution with a stable composition.
Along with these results, some attempts have been made on titanium alloys. Titanium polishing requires high current density to dissolve the anodic titanium oxide layer formed during the electrolytic process. Research works are in progress, in particular to study the influence of additive compounds in the solution, to increase the titanium oxide conductivity, allowing the use of lower potentials.
Aluminum Electroplating
A process using an organic solvent has been extensively studied in the ASETS Defense research program (Advanced Surface Engineering Technologies for a Sustainable Defense). The objective was to substitute cadmium metal deposition with aluminum layers obtained by electroplating for corrosion resistance applications.
This proprietary process based on a relatively old patent from Siemens Company uses a solution entirely formed by organic solvents - tri-ethyl aluminum in solution with toluene. Results are promising but this process is flammable and hazardous, requiring highly controlled parameters to avoid potential explosion. Important security conditions are necessary to avoid any problems with the high quantities of liquid used in the plating bath.
Ionic liquids can be used without explosion risks. Here, the solvent used for aluminum plating in general application is 1-butyl-3-methylimidazolium (BMIM) chloride. In this type of solution (chloride ions), Lewis acid is formed and the general reaction is:
2Cl- + 3AlCl3 --> AlCl4- + Al2Cl7-
The solution composition which gives the optimum result contains a BMIM-Cl-to-AlCl3 ratio of 1-to-(1.5 to 2). Electrolysis is accomplished under nitrogen gas to avoid oxidation and hydration of the compounds in the process. Aluminum electrodeposition is produced at a cathodic current density as low as 0.5 A/dm2. Higher cathodic current densities allow the deposition of fine-grained, bright aluminum.
As for all electrodeposits, surface preparation is very important and it is essential to obtaining good adhesion of the aluminum layers. During the IONMET programme, good deposits were obtained using pure aluminum anodes. Adherent deposits were produced on copper, aluminum, steel and stainless steel substrates. Figure 1 shows the cross section of deposits thus obtained.

Figure 1 - SEM photos of aluminum deposits: (a) surface and (b,c) in cross-section at different magnifications.
Different solutions were tested using other solvents such as ethyl-methylimidazolium chloride (EMIM-Cl-to-AlCl3 :: 1/1.5), which appears to be the best one. These tests also included the deposition of aluminum-magnesium alloys.
The results obtained during these different research studies show that it is possible, using ionic liquids, to realize aluminum deposition by electroplating as well as aluminum alloys. The deposition processes produce aluminum layers with large thicknesses (up to 200 μm). In some applications, by modifying the surface preparation steps, it is possible to produce foils of aluminum which are easily separated from the substrate.
Hard Trivalent Chromium Plating
Alternative processes as substitutes for hexavalent chromium plating are of strong interest due to the high toxicity of Cr(VI) ions. Even if after hard chromium deposition, the toxicity of the deposit is not hazardous as there are no hexavalent chromium ions in the deposits, there remains concern, whether valid or not. A tremendous effort has been spent on research on processes using dry research technology (HVOF, PVD, CVD, thermal treatment, etc.), as well as wet technology, such as electrodeposition or electroless deposition of metals or composites (nickel or cobalt with or without codeposited particles). Currently HVOF and electroless nickel composite processes seem to be the most promising solutions. However, some drawbacks (non-line-of-sight (NLOS) surfaces, hardness, friction coefficient, corrosion resistance, wear resistance, adhesion, etc.) hinder the large development of these methods and their establishment as the universal solution. There have been many attempts over the past decades to use trivalent chromium ions in different chemical compositions in aqueous solution, as well as using different current waveforms, yet none meet the results obtained by Cr(VI) -based electrodeposits.
By mixing choline chloride (2-hydroxy-N,N,N-trimethylethanaminium chloride) and chromium chloride (CrCl3•6H2O) an ionic liquid solution is obtained at a temperature above 35°C (95°F). Using this solution as electrolyte, it is possible to produce hard chromium deposits, such as those shown in Figure 2.
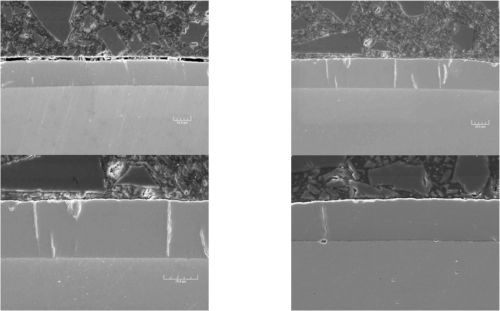
Figure 2 - Cross-sections of ionic liquid-based chromium deposits on steel substrates (SEM).
The operating parameters and deposit characteristics are summarized in Table 1. The deposit hardness can be increased with a 400°C heat treatment, as noted in the table and illustrated in Figure 3.
It is also possible to produce hard chromium deposits with included nanoparticles (Al2O3, alumina or SiC, silicon carbide) and create layers with new tribological properties. SEM photos of composite chromium deposit cross-sections are shown in Figure 4.
Table 1 - Electrolyte conditions and deposit characteristics for an ionic liquid-based Cr(III) electroplating process.
| Solution ratio | CrCl3•6H2O:Choline-Cl :: 2:1 |
| Temperature | 40°C (104°F) |
| Current density | 10-20 A/dm2 (93-186 A/ft2) |
| Deposition rate | 0.7 µm/min (@ 15 A/dm2) |
| Deposition rate | Metallic, shiny |
| Hardness as deposited | 600-700 HVN100g |
| Hardness after 400°C heat treatment | 1,400-1,500 HVN100g |
| Microcracking | Microcracked, with some extending thru Cr layer. |
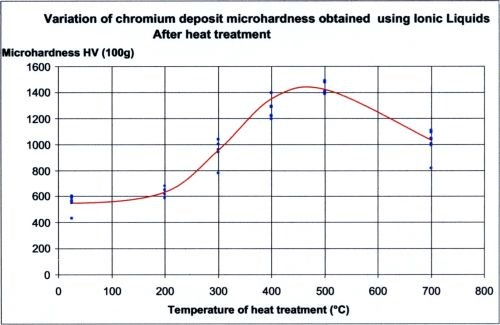
Figure 3 - Variation of chromium microhardness as a function of heat treatment.
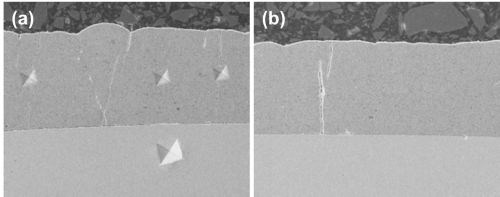
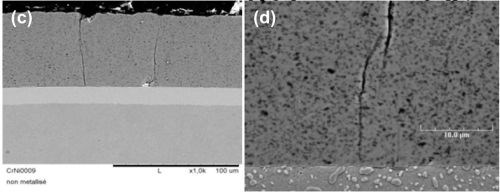
Figure 4 - Cross-sections of composite chromium deposits: (a) with microhardness indentation; (b) showing the general aspect of a 50 µm layer; (c) a composite deposit on nickel layer over steel and (d) the composite at a larger magnification.
The results obtained during our research work are consistent and the solution replenishment procedures have been designed to allow continuous operation of the ionic liquid solutions. However, the low conductivity of the ionic liquid used for chromium plating, (20 mS/cm) versus, for example, 700 mS/cm at 55°C (131°F) for aqueous hexavalent chromium is a drawback. As a result, the adhesion of the ionic liquid-based chromium layers is weaker than that for the hexavalent process. These drawbacks, if not addressed, will have an influence on the use of the ionic liquid process in the future.
Industrial applications
The results from our research work, in our own laboratory and during IONMET program, have shown industrial interest in applying this novel type of electrochemistry to surface treatment and plating. Currently in a number of European research centers, pilot development is in progress. Tanks from 50 to 500 L have been built and the results coming from this pilot work will soon give us information on the industrial potential of these processes.
Ionic liquid-based electropolishing appears to be quite promising and results obtained on a 500 L tank in an IONMET partner jobshop on several industrial scale articles seems to be a candidate for industrial application.
The environmental aspects and the special conditions linked to the using of ionic liquids instead of aqueous solutions (without the anodic and cathodic reaction of water at the electrodes) leads to some very interesting applications in the field of aluminum deposition as a substitute for cadmium layers and for the deposition of new products, such as gallium or indium/gallium alloys (for photovoltaics materials), silicon, selenium, arsenic, etc.
However, for the moment, the high cost of these processes (compared to classical electrodeposition) is an obstacle for future industrial development. Some products used for the aluminum deposition process are very expensive. Nonetheless, one can expect that successful industrial development would lead to increased production volumes, and thus a significant decrease in production costs.
On the other hand, after the cost of the plating solution is taken into account, the real plating cost will be linked only to the deposited metal. After the large investment to make-up the bath, the real cost will only be the cost of metal deposited, not so different from the current cost using aqueous solutions. Further, developing a plating process which would use less tank volume would further reduce the initial investment costs.
Given these considerations, brush plating or other new processes issuing from this method could be the future of plating using ionic liquids. We must explore these possibilities.
Conclusions
- Ionic liquids present a new and interesting development for surface treatment and plating. Electropolishing using ionic liquids have already been developed and pilot tests have been successful.
- The new electrochemistry (out of the Pourbaix aspects) issuing from the new solvent reactions at the electrodes open the way to the development of new plating processes, in particular under different environmental conditions, new metals to be plated (Ga, Si, Al) and modification of the impact of electroplating on the environment (Cr, Cd substitutes).
- The development of processes using reduced solution volumes could be the future of ionic liquid plating technology. Brush plating or like processes show considerable potential in this regard.
Additional Reading
- H. Ohno, Ed., Electrochemical Aspects of Ionic Liquids, 2nd Edition, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2011.
- F. Endres, et al., “Electrodeposition of Nanocrystalline Metals and Alloys from Ionic Liquids,” Angewandte Chemie, Int. Ed., 42 (29), 3428 (2003).
- M.J. Earle & K.R. Seddon, “Ionic Liquids: Green Solvents for the Future,” Pure Appl. Chem., 72 (7), 1391 (2000).
- S.Z. El Abedin & F. Endres, “Electrodeposition of Metals and Semiconductors in Air and Water Stable Ionic Liquids: A Short Review,” Private Communication.
- P. Benaben, “Ionic Liquids: Some Applications in Surface Treatment; Les liquides ioniques: quelques applications en traitement des surfaces” (In French), Traitements & Matériaux, 420, 39, (Jan/Feb 2013).
- A.P. Abbott, et al., “Ionic Liquids Based upon Metal Halide/Substituted Quaternary Ammonium Salt Mixtures,” Inorganic Chemistry, 43 (11), 3447 (2004).
- A.P. Abbott, et al., “Electropolishing of Stainless Steel in an Ionic Liquid,” Trans. Inst. Met. Fin., 83, 51 (2005).
- Proc. 3rd Industrial Workshop - Application of Ionic Liquids in Plating Technology - Munich (Germany) - DGO - 24 March, 2009:
- J. Collins, CTech Innovation, “Electropolishing in Ionic Liquids.”
- K. Ryder - University of Leicester (UK), “Aluminium Electroplating in Ionic Liquids.”
- P. Benaben, M. Boudes, Y. Gamier- Ecole Nationale Supérieure des Mines de Saint-Étienne (France), “Chrome Plating from Cr(III) in Ionic Liquids.”
Related Content
ECOAT24 Attracts Both Serious Electrocoaters and Novices Alike
The conference, held April 2-4, 2024, in Orlando, Florida, provides the ecoating industry with educational sessions, supplier exhibits and networking with colleagues.
Read More10 Ecoat Best Practices
Following this list of guidelines can help to increase the performance, cost effectiveness and quality for your ecoat operation.
Read MoreInstalling an Ecoat Line
Thinking of investing in electrocoating capabilities? George Lovell, coatings plant manager for Lippert, discusses considerations you should keep in mind as you add your ecoat line.
Read MoreCoatings Plant Evolves with Market Trends
Expanding its focus from exclusively serving the RV industry, one of this company’s stand-alone coatings plant has successfully extended its services to additional markets.
Read MoreRead Next
Episode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More


















.jpg;maxWidth=300;quality=90)








