Current Density Revisited
Question: Anodizers constantly raise the question of anodizing (sulfuric acid) by current density vs. voltage.
Question:
Anodizers constantly raise the question of anodizing (sulfuric acid) by current density vs. voltage. They may not always ask directly, but when they’re having problems achieving correct or consistent coating thickness, they’re wondering why this is happening. Although nearly all anodizers have heard of running by current density, many are unsure exactly how to do it. There are also some who figure that would to be more work. Can you address this topic? B.G.
Answer:
In reviewing almost nine years of columns, I can only find one other article that addresses anodizing by current density (May 2005, “Hardcoat Anodizing Controls”). The topic begs to be addressed again.
Some anodizers I have spoken with have thought that anodizing by current density only applies to hardcoat (Type III) anodizing. Not true. Using the constant current density method of anodizing can make your life as an anodizer so much easier—and the quality and consistency of your work better.
As I stated a few years ago, if a known quantity of current is passed through parts under controlled conditions of chemistry and temperature, the time to anodize to the desired coating thickness can be closely calculated. Chemistry and temperature are important parts of this equation, so it becomes important to closely control both of these variables.
This is what is meant when we talk about having the process under control. The process is either under control or out of control. There is no in between. You set the parameters for control of the process in your own anodizing line. Once you decide on realistic control limits for chemistry, with the proper frequency of bath analysis and “honest” chemical additions, the bath can be controlled within those limits by using the principles of Statistical Process Control (SPC) and plotting the results of your analysis on a simple “X bar R” chart. Post it where the anodizers can see it every day. This can be done manually, or there’s software available for download that’s easy to use and moderately priced.
Temperature control in the anodizing line is sometimes a problem. This is where many anodizing processes can get derailed. Tanks that need to be cooled are usually more of a problem than tanks that are heated. This is because, commonly, the cooling system is either undersized or not designed correctly. It’s important to control all process bath temperatures within a specified range.
If the temperature of heated tanks varies widely, it is possible, even likely, that inconsistent results will be attained in the quality and/or appearance of the parts. Control of the anodizing bath temperature is most critical, and a reasonable control range is plus or minus 2oF (1oC). This degree of control is important over the entire range of the anodizing cycle and the cooling system should be sized and designed to achieve this under a constant “full load” condition.
There are some loads being anodized out there that seem to defy the practical limits of calculating the surface area of the parts on these loads. These might be one-off, irregularly shaped parts and, at best, small quantities are involved. Probably more than 95% of all parts anodized repeat and are capable of having their surface areas easily calculated. It’s these parts that we’re talking about. Yes, it’s more trouble to do this initially, but the results are worth the effort. Whether the surface area is already available on computer-generated parts drawings, or a tape measure and hand-held calculator are used to estimate the surface area, knowing the total surface area to be anodized on each load will lead to load-to-load consistency. Controlling the bath parameters leads to better batch-to-batch reproducibility.
While hardcoat (Type III) finishes are generally processed using 24–40 asf (2.6–4.3 asd), Type II, clear or dyed “commercial” and decorative parts are usually processed using 10–18 asf (1.1–1.9 asd). Obviously, the load must be sized for the rectifier capacity. For example, if you have a 5,000-A, 24-V rectifier and you want to anodize at 12 asf to achieve 0.35–0.40 mils (8.75–10 µm) coating thickness, or any coating thickness, the following formulas may be used to calculate the required anodizing time:
We also know that at 12 asf (1.3 asd), the maximum surface area we can put on this theoretical 5,000-A load is:
Note that the surface area of the load is inclusive of the rack area. If the rack is titanium, then it does not have to be taken into consideration.
For the 95% of parts that are repeats, keep a record of their surface areas. One-off parts may have a computer generated part-drawing and the surface area might be calculated by the software. That makes it pretty easy.
How do we get the rectifier to run by amperage instead of voltage, you ask? Glad you asked that question because it’s really easy to do this. Here’s how:
- While the rectifier is in standby with no load in the tank, turn the voltage pot all the way to the right. This sets the voltage at maximum and essentially takes voltage out of the control circuit.
- Make sure the amperage pot is turned to zero, or all the way to the left.
- If you have digital controls instead of analog, input the maximum voltage output capability of the rectifier. This takes the voltage out of the control circuit. Make sure the amperage is set to zero. (It will be zero if there are no parts in the tank.)
- Put the load in the tank and turn on the rectifier.
- Using the amperage control, (or automatic ramping control) turn the amperage up to the full amperage setting of the load as determined by the desired current density multiplied by the total surface area of parts (and racks, if aluminum). This gives the amperage of the load.
- Use the formulas above to determine how long to anodize.
- When the anodizing cycle is finished you can rinse the parts, dry off a small area with a clean rag and measure the coating thickness. Or wait until after the load is sealed and check the coating thickness in the unracking area.
- If the coating is not quite what you want, adjust the anodizing time up or down slightly.
If you follow this procedure and don’t achieve the correct coating thickness, there are a number of things you can check:
- Check your math.
- Check the surface area calculation of each part number. Perhaps the actual surface area is slightly different than what you calculated.
- Check the calibration of the amperage and voltage meters. (That’s a routine maintenance and calibration item that should be performed against a standard at least every 6 months.)
- Make sure chemistry is within the control range.
- Make sure the temperature is held within the control range throughout the duration of the anodizing cycle. If conditions in the tank change from the beginning of the cycle to the end of the cycle, it is possible that you could see a variation in the results.
Some things to remember about anodizing:
- The amperage (current density per unit of time) builds coating and determines what the final coating thickness will be.
- Voltage is the force required to push the amperage through the parts under a given set of processing conditions.
The rules above deal with amperage and not voltage because voltage, in most cases, is incidental. If you make the bath more conductive by raising the concentration and/or the temperature, but still run at the same current density, the voltage associated with that particular current density will be lower (maybe only slightly).
Conversely, if you make the bath less conductive by lowering concentration and/or temperature, but keeping the same current density, the associated voltage will be only slightly higher to achieve the required current.
So, you can see that if you process by voltage only, the current density will vary according to the bath conditions of concentration and temperature. This will change the resulting coating thickness—as the anodic coating thickness grows, if the voltage is fixed, the current may drop off anywhere from slightly to a lot. This causes the load to run longer in order to achieve the desired coating thickness because the current density drops as the load continues to run. It becomes self-defeating when heavy coating thicknesses (required by many hardcoat specifications) take 60 to 90 minutes or longer to achieve, or sometimes can’t be achieved at all.
One last note. Traditionally, there are some anodizing processes that are run by voltage. Two commercial anodic finishes that normally run by voltage are chromic acid anodizing (Type I) and phosphoric acid anodizing. Even these can be run by current density, but they usually are not, simply because an exact coating thickness is not the objective for these finishes.
Related Content
Finisher’s ‘Top Shop’ Status Attracts Business
This competitive California finisher made it a goal to become a PF Top Shop. After earning the recognition, the company experienced an immediate increase in business and a challenge to obtain certifications.
Read MoreUnderstanding and Managing White Spots on Anodized Aluminum
Having trouble with spotting defects when anodizing? Taj Patel of Techevon LLC offers a helpful overview of the various causes of white spots and potential solutions.
Read MoreChicago-Based Anodizer Doubles Capacity, Enhancing Technology
Chicago Anodizing Company recently completed a major renovation, increasing its capacity for hardcoat anodizing and Type II anodizing.
Read MoreRead Next
Episode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More





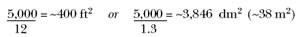







.jpg;maxWidth=300;quality=90)













