Nanoscale Characterization of Thin Immersion Silver Coatings on Copper Substrates
The rather long history of silver plating, both by electrodeposition and via immersion plating, started with the cyanides. Though such compounds are rather hazardous, they are still used today for many metal plating tasks in the microelectronic industries. New trends, aim at replacing the cyanide. Using new complexing agents and additives in the newer industrial immersion silver baths, there should be less concern that immersion silver processes would be inappropriate or less advantageous than gold in high-tech applications in the microelectronic industries.
by
Tamás I. Török,* Éva Kun & Dániel Sós
University of Miskolc
Miskolc, Hungary
and
Attila Csik, József Hakl, Kálmán Vad, László Kövér, József Tóth & Sándor Mészáros
Institute for Nuclear Research
Hungarian Academy of Sciences
Debrecen, Hungary
Editor’s Note: This paper is a peer-reviewed and edited version of a presentation delivered at NASF SUR/FIN 2014 in Cleveland, Ohio on June 9, 2014. A printable PDF version is available by clicking HERE.
ABSTRACT
Microelectronic-grade copper foils were immersion silver plated in a home-made non-cyanide alkaline silver nitrate - thiosulfate solution and in two commercially available industrial baths via contact reductive precipitation. The concentration depth profiles of the freshly deposited silver layers were afterwards analyzed at nanoscale resolution by means of secondary neutral mass spectrometry (SNMS) and glow discharge optical emission spectroscopy (GDOES). The thickness of the deposited silver layers obtained was in the range of 50 to 150 nm, depending on the parameters of the immersion procedure. Slight contamination of sulfur from the thiosulfate bath was detectable. Traces of chromium and sodium could be observed as well around the interface between the copper substrate and silver deposit. The results also indicate that storage for longer time in air, especially at higher than ambient temperatures, induces a kind of aging effect in the deposited layer, changing its composition. The samples were also analyzed by x-ray photoelectron spectroscopy (XPS) to identify the chemical state of the silver.
Keywords: silver, copper, displacement, immersion coating, GDOES, SNMS, depth profiles
Introduction
The rather long history of silver plating, both by electrodeposition and via displacement reactions (so-called immersion plating), using aqueous solutions of soluble silver compounds, started with the cyanides. Though such compounds are rather hazardous, they are still used today for many metal plating tasks, even in the microelectronic industries. New trends, however, aim at replacing the cyanide anions with other complexing agents (Table 1) in both electroplating and immersion silver plating. In some cases, the latter technique is becoming favored as a surface finishing alternative to protect the surface of conducting copper layers from excessive oxidation, e.g., before lead-free tin base soldering in many applications, such as printed circuit boards (PCB) (Table 2).
Table 1 - Non-cyanide complexing agents proposed to replace cyanide in silver plating baths.1
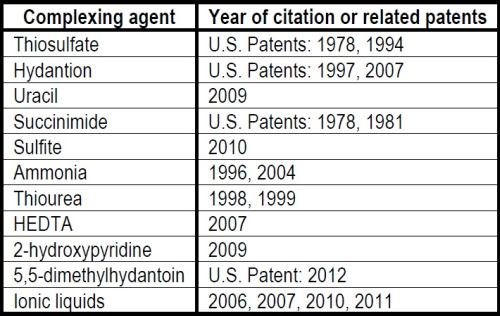
Table 2 - Major surface finishing techniques used in soldering by the microelectronics industry.2
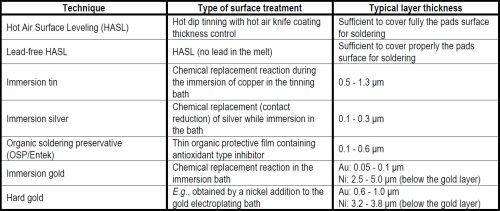
For any high quality immersion silver plating procedure, the commercial proprietary immersion silver baths, in addition to the silver complex, might normally contain several other important additives, such as buffering agents to control the pH, corrosion and/or anti-tarnishing inhibitors, and also some optional ones, such as surface active or wetting agents, grain refiners or brighteners, defoamers, etc. For any successful plating operation, there are operational parameters (temperature, hydrodynamic properties, possible contaminants, etc.) which all should be optimized and controlled meticulously together with the proper chemically active surface condition of the copper substrate itself.
In view of the above mentioned complexity of the contact reductive precipitation of silver onto copper, in our laboratory experiments, three different immersion plating baths were tested in laboratory deposition trials. Prepared thin silver deposits were analyzed in depth on the nanoscale by means of two surface sputtering elementary analytical techniques: (1) secondary neutral mass spectrometry (SNMS) and (2) glow discharge optical emission spectrometry (GDOES), in order to reveal the major clues to obtaining high quality silver deposits on copper via immersion plating.
Experimental (Materials and methods)
Copper foils
The copper substrates consisted of thin copper sheets with an average surface roughness of Ra ≈ 0.24 μm on the polished side were used after proper cleaning and etching. The thickness of the copper foils was approximately 90 μm.
Immersion silver plating methods
In addition to our silver plating method with home-made solutions (Type A), a complete set of industrial silvering solutions (Type B) was received from a PCB manufacturer and another one (Type C) was prepared in our laboratory from concentrates purchased from a chemical supplier. However, because of proprietary considerations, the exact chemical composition of the solutions is not known by us. The major characteristics of the three immersion plating solutions are summarized in Table 3.
Table 3 - The three immersion silver plating solutions used in the laboratory experiments.
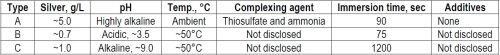
Immersion silver plating with home-made thiosulfate solution (Type A). For the preliminary laboratory deposition trials, an old and rather short recipe3 (Type A) was chosen, based on the following experimental procedure:
1. Surface cleaning:
- Degrease.
- Mechanically scour.
- Rinse with distilled water.
- Ultrasonic treatment in acetone for 25 min.
- Rinse with distilled water.
2. Chemical etching:
- Etch in 2% HNO3 solution for 3 min (max. 7 min).
- Rinse with distilled water.
- Rapid dry.
3. Silver immersion plating:
- Dip in the immersion silver bath for 1.5 min max.
4. Washing with water:
- Rinse with distilled water.
- Rapid dry
- Sonication (ultrasonic treatment) in distilled water for 2 min
- Rapid dry.
To prepare the Type A immersion silver bath, 0.75 g silver nitrate (AgNO3) was placed in a beaker, which was filled with 70 mL distilled water and stirred with a magnetic stirrer at room temperature. When the silver nitrate dissolved, 10 mL of 25% ammonia solution was added. Thereafter, 10.5 g of sodium thiosulfate (Na2S2O3) was gradually mixed into the solution under stirring. Finally, the prepared solution became colorless and transparent.
Immersion silver plating with industrial solution Type B. The laboratory treatment included the following major steps:
- Etching of the copper specimens/substrates in sulfuric acid (~6.5%) also containing hydrogen peroxide (~5.5%).
- Pre-dip treatment for surface activation/conditioning
- Immersion plating in a complexed silver/organic system.
An etching time of 30 sec at a solution temperature of 40°C was found to be optimal with a measured copper surface loss corresponding to about 1.2 μm dissolution. Pre-dipping at ~40°C and silvering at ~50°C lasted somewhat longer (40 sec and 75 sec, respectively), which was found to be close to optimal. All solutions were stirred. We found that rinsing and drying were very important, especially after the silver plating step.
Immersion silver plating with industrial solution Type C. The laboratory treatment included the following steps:
- Clean with a proprietary solution** at 50°C for 4 min.
- Rinse with distilled water at room temperature for 45 sec.
- Activate surface with copper activator for 3 min.
- Rinse with distilled water.
- Etch the surface in 7% H2SO4 solution for 45 sec at room temperature.
- Rinse with distilled water.
- Immersion silver plate at 49°C for 20 min.
- Rinse with distilled water.
- Etch the surface in 7% H2SO4.
- Rinse with distilled water.
- Neutralize the silver deposit with proprietary anti-tarnish solution*** at 49°C for 1.5 min.
- Rinse with distilled water.
- Dry.
All surface treatment solutions noted above were prepared according to the methods specified by the chemical supplier.
Surface analytical techniques
By immersion plating one can produce only rather thin (100-300 nm thick) deposits. Therefore the elementary concentration depth profiles were measured by GDOES (Glow Discharge Optical Emission Spectrometer, type GD Profiler 2, France) and SNMS (Secondary Neutral Mass Spectrometer, type INA-X from SPECS GmbH, Germany). In the case of the depth profiles, the horizontal axis represents the sputtering time of argon plasma which is proportional to the crater depth of the specimen. The sputtering time was converted to the depth of the sputtered craters using profilometers (MarSurf M400 and Ambios XP-I).
Results
Preliminary experiments with thiosulfate bath (Type A)
During our studies, many approaches to develop a sound plating procedure were tried. In our first work, some light yellowish discoloration was often observed on the freshly immersion silver-plated specimens (Fig. 1). This discoloration was intensified with increasing time (over a few hours). When the silver-plated specimens appeared as copper-colored, in all the cases, some copper was found to be present on the surface of the deposited silver, as detected by the GDOES analysis. Later on, the optimization of the process resulted in a more durable, matte silvery surface. When the cleaning and degreasing of foils were performed with a sponge, the silver-treated copper foils became copper-colored within only a few hours. Subsequently, we used a scouring pad during the cleaning process, whereupon the silver color became durable.
It was also observed that whenever a properly cleaned copper test foil was dipped into the immersion silver bath, a continuous solid silver layer was built up within one to two seconds. At first, the silver layer peeled off because of the long immersion time in the silver solution. Through repeated experiments the optimum process time was set finally to 90 seconds.
To find the reason for the discoloration, we examined many chemical and/or transport processes that could induce the diffusion of the atoms within layers. We followed the concentration changes in the silver-plated copper specimens during a three-week period of ambient annealing (at intervals of 20 min, 1 hr, 2 hr, 4 hr, 1 days, 2 days, 4 days, 1 week and 3 weeks). The main curves are shown in Fig. 2. It is notable that while the position of copper peaks remained unchanged, the silver layer seems to diffuse into the bulk copper with time.

Figure 1 - Immersion silver plated copper foils prepared without prior scouring: (L) promptly after coating; (R) one hour later.
Compared to the GDOES technique, the SNMS method has lower sputtering rate (~0.1 nm/sec) and it provides for a more detailed analysis of the silver layers. The SNMS depth profile of a plated sample stored in air for three weeks was measured and the contaminants were detected in the layered structure formed as well (Fig. 3). Sulfur runs together with the silver, while sodium is enriched on the silver surface and at the Ag/Cu interface. These observations are associated with the anionic/cationic nature of the constituents (with respect to silver) during the immersion plating process. The Ag/Cu interface is partly contaminated with oxygen. Oxygen can be found on the silver surface as well. However, its magnitude is partly affected by the instrumental background. The chromium detected originates most probably from the surface treatment of the copper foils.
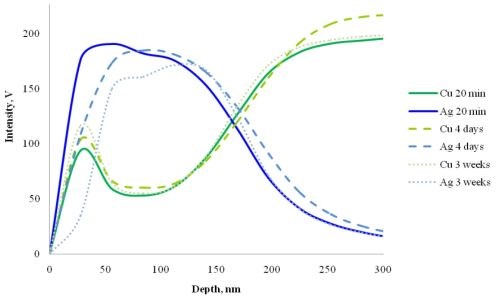
Figure 2 - GDOES depth profiles of single immersion silver-plated copper foils (average of five parallel measurements on different samples).
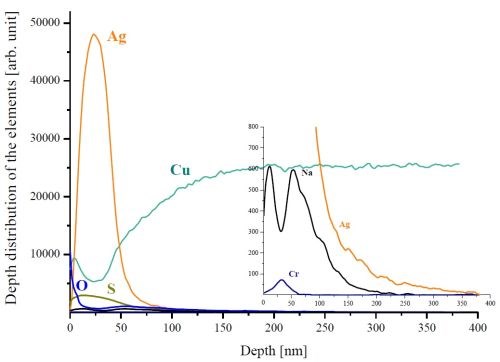
Figure 3 - SNMS depth profile of a silver-plated copper foil stored in air for three weeks.
The two industrial immersion plating baths were tested in a number of laboratory experiments and many of the freshly silver-plated copper specimens (foils) were examined via GDOES analysis (Fig. 4). Argon sputtering removed the thin silver deposits rather quickly (The sputtering rate of GDOES is >100 nm/sec), after which the copper substrates were eroded further by the argon plasma until the detected silver intensities almost diminished and the copper intensities reached the same plateau. As the substrates consisted of the same material in both cases (bath Type B and C) the significantly lower silver intensities recorded with the Type C specimens indicate structural differences in the silver coatings. This variance is reflected in the difference of the Ag/Cu interface thicknesses, which in turn is connected to the coating surface roughness. As a consequence, the Type B coating can be regarded as more compact and robust to environmental degradation.
SNMS investigations were also performed for these samples. It was found that both Type B and C silver layers were clean. Only a small amount of carbon was found in the deposited silver layer and sodium and sulfur accumulated at the Ag/Cu interface (Fig. 5). It must be noted however, that contrary to the sample prepared from the Type A bath, the sulfur was only found at the Ag layer/substrate interface and not in the whole entire silver layer. No chromium was visible at the Ag/Cu interface in these samples.
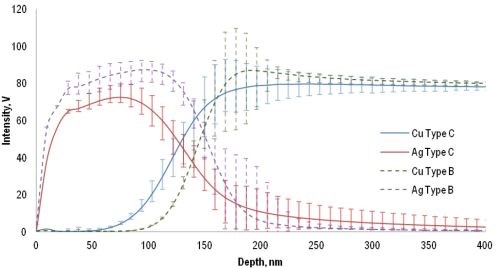
Figure 4 - GDOES depth profiles of B and C type silver plated specimens (average, minimum and maximum values obtained from ten parallel measurements).
To confirm these results, an x-ray photoelectron spectroscopy (XPS) spectrum was acquired at a depth of 25 nm in the silver layer (Fig. 6). The targeted depth was attained by sputtering in the SNMS chamber. The sample was subsequently transferred in a common vacuum system into the XPS chamber. Because the SNMS and XPS instruments were connected through the vacuum tube, it was possible to move the sample under high vacuum without contaminating the freshly sputtered surface. The narrow scan of the Ag 3d peak (Fig. 7) fits well with the spectra of the silver reference sample. This confirms the SNMS results, namely that the silver layer is pure, no contamination exists in the layer (e.g., oxide, sulfate), and the silver is present in metallic state. The binding energies of the Ag 3d lines agree within ± 0.1 eV with the NPL (UK) and NIST (USA) standard values of 368.25 eV. Further, he energy difference between the Ag 3d and the Ag MNN Auger-lines agree within ± 0.1 eV with the mentioned standard values. Both of the measurements (sample and standard) of the Ag 3d lines were made in the same instrumental set up of the XPS machine. During the XPS measurements the vacuum level was in the 10-10 mbar range.
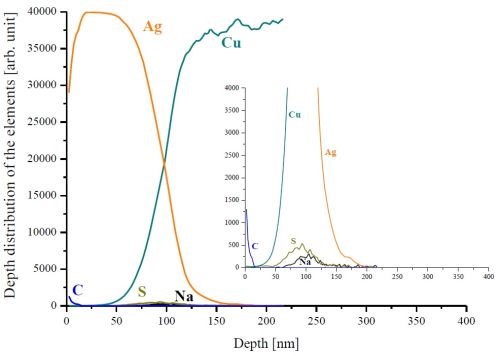
Figure 5 - SNMS depth profile of one of the specimens coated using the Type C silver plating method.

Figure 6 - XPS survey scan recorded at a 25-nm depth in the silver coating of the same Type C specimen as in Fig. 5.
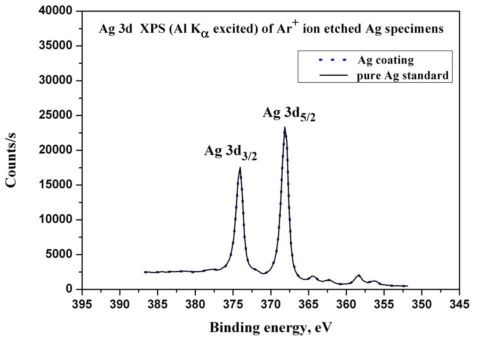
Figure 7 - Comparison of the XPS narrow scans of the Ag 3d peaks measured in the case of the silver coating and the pure silver reference samples.
Discussion
At first glance, immersion silver plating might be thought of as a quite simple process to cover the surface of pure copper substrates (e.g., pads of PCBs). In principle, it works by a simple chemical displacement reaction between the solid copper and Ag+ cations in the plating solution. Such solutions should at least contain a soluble salt of silver and some necessary additives to control, first of all, the kinetics of the contact reductive precipitation of silver onto the copper substrate which is concurrently dissolving into the plating bath. In reality, there are many parameters which have to be controlled during the plating process in order to produce an even and sound (nonporous) layer with good adherence. All these facts explain why even the basic chemical constituents of the immersion silver plating solutions are still under continuing development.4
For our laboratory immersion silver plating experiments, we first chose an alkaline non-cyanide silver plating “recipe” with very limited accessible information.3 Accordingly, the entire immersion plating methodology had to be developed during our laboratory trials. Nonetheless, several sets of thin silver plated specimens were successfully prepared, although many of the silver coated specimens showed some kind of discoloration. It is well known that the number of factors related to color perception by the human eyes can be rather high, around fifteen.5 In the case of pure silver metal, its light absorption peak lies in the ultraviolet region, and as a result, silver maintains high reflectivity evenly across the visible spectrum and we see it as a pure white.6 However, tiny globular silver grains, e.g., those produced in transparent polyacrylamide gels, which contain different proteins as well, appear blue (with grain diameters of 40-100 nm), then yellow (21-39 nm) or brown (17-35 nm), these peculiar phenomena being described in detail in 1988.7 Since then, the coloring effects of metal nanoparticles, e.g., the plasmon resonance mechanism, have generated much attention.8
During our laboratory experiments, when we first noticed the discoloration of our immersion silver-coated copper specimens stored in air, the aspects of the formation of surface colors noted above were all quite helpful in trying to explain the situation in our case. The surface corrosion (often called tarnishing) of silver, especially in contaminated air, is also well known among metal processors, silversmiths and art collectors,9 and the thermodynamic stabilities of the most common corrosion products of silver and copper, in principle, confirm those observations (Fig. 8). Concerning the sulfur-copper reaction and taking into account the results of SNMS depth profiling (Fig. 3), we can conclude that this causes color changes observed on the sample prepared from the Type A bath.
It is seen from the data (calculated by the HSC Chemistry 5.0 Thermodynamic Database and Software) given in Fig. 7, that both oxides of copper are quite stable, while silver should form sulfides more readily than oxides. Further, when the more noble silver metal is in contact with copper, as in our case, there is also an additional electrochemical driving force towards the anodic oxidation of copper. Moreover, if the circumstances are favorable (e.g., under moist conditions), the galvanic corrosion of copper should occur first. However, modern silver plating baths and procedures are so well developed - as observed with the two industrial baths in our experiments - that users should not be overly concerned, provided all the pre- and post-treatment and other procedures and technological parameters are well controlled.
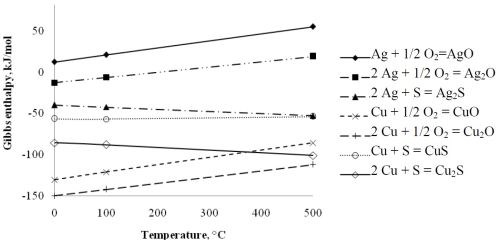
Figure 8 - Standard Gibbs enthalpies of formation for the oxides and sulfides of silver and copper, respectively, as a function of the temperature.
Conclusions
A non-optimized simple immersion silver plating bath, such as our home-made non-cyanide and highly alkaline bath was found to be unsuitable for high-tech industrial applications, although we could gain quite useful information about the weak points of immersion silver plating on copper during our preliminary experiments. First, the rather thin (around 50-150 nm) and porous silver deposits did not last long without surface discoloration, partially due to the incorporation of sulfur from the thiosulfate bath itself and migration of the constituent silver and copper atoms. Second, proper surface cleaning and bright etching were found to be crucial steps in developing a sound and smooth silver deposit. Using new complexing agents and additives in the newer industrial immersion silver baths, there should be much less concern that immersion silver processes would be inappropriate or less advantageous than gold in high-tech applications in the microelectronic industries.
Acknowledgement
The research work presented in this paper was based on the results achieved within the TÁMOP-4.2.1.B-10/2/KONV-2010-0001 project and carried out as part of the TÁMOP-4.2.2.A-11/1/KONV-2012-0019 project in the framework of the New Széchenyi Plan. The realization of this project is supported by the European Union, and co-financed by the European Social Fund.
References
1. A.M. Liu, et al., Scientific Reports, 4, 3837 (2014); (DOI:10.1038/srep03837); http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3899642/ (last accessed 01/28/2015).
2. http://www.epectec.com/downloads/surface-finishes.pdf , Epec, LLC, New Bedford, MA (last accessed 01/28/2015).
3. S. Hirsch & C. Rosenstein, Metal Finishing, 100 (Supplement 1), 421-424 (2002).
4. Z. Wei, D. Tang & T.J. O’Keefe, China Particuology, 3 (5), 271-274 (2005).
5. K. Nassau, The Physics and Chemistry of Color: The fifteen causes of color, 2nd Ed., Wiley-Interscience, Hoboken, NJ, 2001.
6. M. Douma, Curator: Cause of Color (2008); http://www.webexhibits.org/causesofcolor (last accessed 01/28/2015).
7. C.R. Merril, et al., Proc. Natl. Acad. Sci. USA, 85 (2), 453-457 (1988); http://www.ncbi.nlm.nih.gov/pmc/articles/PMC279568/, (last accessed 01/28/2015).
8. E. Petryayeva & U.J. Krull, Analytica Chimica Acta, 706 (1), 8-24 (2011).
9. M. Inaba, Conservation Journal, 18 (1), 9-11 (1996); http://www.vam.ac.uk/content/journals/conservation-journal/issue-18/tarnishing-of-silver-a-short-review/, (last accessed 01/28/2015).
About the authors


Related Content
Calculating Applied Media Force During Vibratory Finishing
What appear to be identically set-up vibratory bowls will finish identical loads of parts in varying time cycles. This paper offers a new technique to better predict what the operator will produce, by measuring the force applied to the parts. It is the efficiency of that force which controls the efficiency and speed of the refinement cycle.
Read MoreDevelopment of a Novel Hexavalent-Chromium-Free Aluminum Sacrificial Paint
Hexavalent chromium is a known carcinogen, repro-toxin, and mutagen. Its elimination is of high importance to the aerospace industry, which has struggled to find high performing alternatives. Legacy aluminum sacrificial paints have traditionally utilized hexavalent chromium to prevent corrosion and coatings which are equal to or better than have been difficult. This first paper discusses the novel process from the supplier point-of-view.
Read MoreConnect, Collaborate and Contribute to the Industry at SUR/FIN 2024
Atlanta, Georgia, is the home to this year’s NASF SUR/FIN conference and trade show.
Read MoreHexavalent-Chromium-Free Aluminum Sacrificial Paint Validation
Hexavalent chromium is a known carcinogen, repro-toxin and mutagen. Its elimination is of high importance to the aerospace industry, which has struggled to find high performing alternatives. Legacy aluminum sacrificial paints have traditionally utilized hexavalent chromium to prevent corrosion and coatings which are equal to or better than have been difficult. This second of two papers discusses the hexavalent-chromium-free process from the user point-of-view in terms of the process validation work by Rolls Royce Corporation.
Read MoreRead Next
Episode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More















.jpg;maxWidth=300;quality=90)












