Science of Successfully Anodizing Die Cast Substrate
Die castings pose some of the most challenging problems in anodizing. This paper provides some explanations by tying together metallurgical science with anodizing practice.
Die castings pose some of the most challenging problems in anodizing. The finish can be too thin, non-uniform and/or have an unfavorable appearance. These are common problems with a variety of practical solutions; they are easy to recognize, but in many instances, the source for the problem remains unknown. Critical to solving the problems of anodizing die castings is understanding the die cast substrate and the impact of surface condition, alloy composition, casting quality and microstructure on the anodizing process. Substrate quality issues are just as important, maybe more so, than anodizing conditions and technique. Certain optimum anodizing conditions may be used in some cases to help overcome less than advantageous metallurgical conditions. These include well known processing tools such as various pretreatment chemistries, higher anodizing bath concentration, and higher bath temperatures. These, and other recommended solutions are not successful in every case; sometimes trial and error testing on actual production parts must be done to find the best processing techniques. Through the use of actual case studies that provide real-life solutions in terms of anodizing theory and interfacial science, this paper provides some explanations by tying together metallurgical science with anodizing practice.
INTRODUCTION
Review of several questions received in Products Finishing Magazine over the years 2001 - 20091-8 determined generally consistent problems with anodizing die castings. The problems can be summarized as follows: 1) the anodic oxide finish is too thin to meet the necessary design specification or much thinner when compared to corresponding components manufactured from wrought processes; 2) the finish appearance is unfavorable: hazy, muddy, “not black enough”, and/or not uniform; and, 3) the corrosion resistance of the anodic oxide is insufficient. Review of recommended anodizing solutions to the various problems with anodizing cast alloys determined that they don’t always work, indicating that there are factors other than the anodizing process that impact the anodic oxide finish quality.
Consideration given to solve these rather easy-to-identify problems has illuminated four broad cause areas for discussion: 1) alloy selection, 2) substrate quality, 3) surface treatment, and 4) anodizing process parameters. Of the four, perhaps the metallurgical factors that impact substrate quality: alloy composition, casting quality, microstructure and surface quality are dominant in determining anodizing conditions, technique and quality.
This paper covers each of the four cause areas by discussing, in limited detail, the impact each has on the interface from which the anodic oxide originates and grows. Theoretical scientific reasons for why the problems occur and why most solutions succeed and some fail are presented. The limitations as to what can be done from an anodizing standpoint to overcome the metallurgical condition of a cast substrate are presented not as an excuse, but as a call for understanding and communication between metal finishers, component designers who would like to use die castings, and the casting houses who provide the castings in order to optimize product and process and to increase the use of anodized cast aluminum components.
Alloy Selection
Aluminum die castings have been commercially available since the beginning of the 20th century. Castings are used for a variety of applications, from decorative sculptures and jewelry to automotive pistons and engine blocks.
Die casting is a versatile process for producing engineered metal parts by forcing molten metal under high pressure into reusable steel molds. These molds, called dies, can be designed to produce complex shapes with a high degree of accuracy and repeatability. Parts can be sharply defined, with smooth or textured surfaces, and are suitable for a wide variety of attractive and serviceable finishes.9
First and foremost, cast alloys are formulated for castability, followed by mechanical properties and structural integrity. Alloys are formulated for maximum fluidity, minimum gassing when molten, and ability to remove the casting from the mold when solid. Mechanical properties are insured first by the integrity of the casting, therefore, the casting alloy and process are designed to minimize porosity and maximize surface integrity. Actual mechanical properties of strength, hardness, and resistance to wear and fatigue are produced metallurgically in aluminum castings two ways: (1) by solid solution hardening; that is: by the substitution of aluminum atoms with alloying atoms in the aluminum crystal structure and (2) by precipitation hardening: the dispersion of second phase constituents or elements in solution and precipitating them out as small intermetallic compounds, incoherent with the microstructure, which inhibit material deformation.
Cast components have limited ductility and can be brittle; therefore, castings are not usually meant for subsequent deformation processing. Other than minimal finishing processes such as machining, a casting is typically produced to function near net shape. Rarely, if ever, is a cast alloy designed for subsequent surface treatment, especially anodizing, because, alloying elements do not anodize.
Because cast components are produced to function near net shape, castings can be alloyed beyond what is typical for wrought products; that is, additions of other elements are at a higher per cent than the additions for alloys intended for extruded, rolled or deep drawn product (up to 16% total alloy content for castings vs. up to 8% for wrought alloys). As such, cast alloys are metallurgically more complex than their wrought counterparts; increased alloy additions produce correspondingly higher levels of solution phases, intermetallic compounds and precipitates. Castings, therefore, in addition to their strength and fatigue resistance, exhibit more complex surfaces, with less free aluminum, which make them more difficult to anodize.
Cast Alloy Designations
Table I presents the compositions of some commonly specified aluminum die cast alloys according to their American, European and Japanese designations10. The alloy chemistry and the casting process affect the level of microstructural homogeneity, the defect population and therefore the variation of chemical potential across a cast component surface. It is important to understand the nature of the surface and therefore the interface between the component surface and the anodizing electrolyte such that anodizing process parameters can be modified to effect optimum oxide growth.
Silicon is the alloying element that essentially makes the commercial viability of the high volume aluminum casting industry possible. Silicon content between 4% to the eutectic level of 12% reduces scrap losses, permits production of much more intricate designs with greater variations in section thickness and yield castings with higher surface and internal quality (forms sound outer surface layer), because of this, aluminum-silicon cast alloys can be used in applications where pressure tightness is a requirement. These benefits derive from the effects of silicon and aluminum molten mixtures which exhibit increased fluidity, reduced cracking and improved feeding to minimize shrinkage porosity. Alloys with the eutectic composition (Al-12%Si) exhibit highest fluidity during casting.
Copper, Magnesium and Zinc are the most important secondary alloying elements which impart fluidity during casting and various phases which impart mechanical properties such as strength, corrosion resistance and fatigue resistance. Alloying elements have varying characteristic solubility in aluminum, and how they mix, stay in solution or precipitate as intermetallic compounds impact the cast microstructure. All die cast components contain some level of both phenomena, to varying levels depending upon alloy chemistry, casting process and any post-cast heat treatment (whether accidental or actually imposed).
Table 1: Compositions of Some Commonly Specified Aluminum Die Cast Alloys*
;ll';l';
*Data taken from ASM Specialty Handbook, Aluminum and Aluminum Alloys, ASM International, Materials Park, OH, 199310.
Substrate Quality
Controlling Cast Microstructure
Cast microstructure dictates the component quality. Because of the rapidity of the die casting process, microstructures are finer than components cast by other methods. The size and distribution of the various phases, compounds and precipitates hinge on: alloy chemistry, which includes the addition of elemental modifiers to the alloy chemistry; the casting method; the method and speed by which the casting is cooled, and whether it is heat treated (tempered) after it is finished.
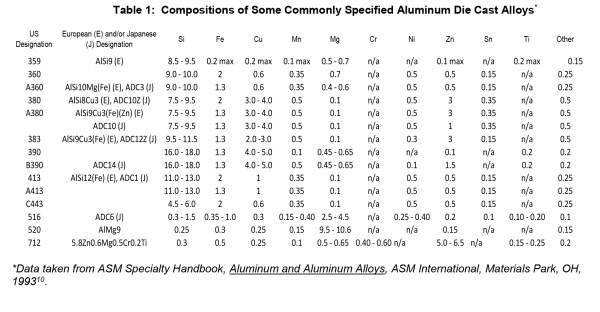
Die casting dies are designed with vents and cooling lines to facilitate rapid cooling. In addition, components can be ejected into a cooling tank to rapidly quench finished components. To keep microstructures fine with complex alloys, the rate of cooling must be as fast as possible, in order to keep as much of the elements in solution as possible. If cooling media is not turned over as rapidly as components are loaded, inadequate cooling can develop coarse cast microstructures. Precipitation and growth of intermetallic compounds, phase formation and grain coarsening will occur if the casting is slow cooled, or allowed to remain at a high enough temperature after ejection from the die. Latent heat retained in castings will cause tempering effects that are manifested in the microstructure by the growth large primary silicon crystals and coarse precipitates. Microstructural effects on the surface are developed by the anodizing process. See Figure 1.
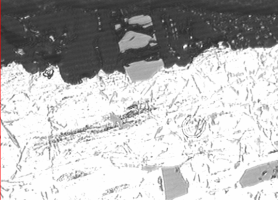
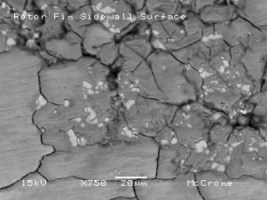
Figure 1: Metallographic cross section of anodized die cast alloy AlSi9Cu3Fe (similar to alloy 383). In the micrograph on the left, large hypereutectic primary silicon platelets from the microstructure and smaller eutectic needles are incorporated into the anodic oxide. In the SEM photomicrograph on the right, the surface of the component is documented, showing the cracks dotted with white-appearing silicon as well as copper deposits appearing as darker-gray haze across the surface and concentrated in areas near the cracks.
Because die casting is a closed process, the amount of gas dissolved in the die cast melt can precipitate out as porosity. This is typically avoided by degassing the melt prior to injection. However, in the event that porosity is close to or intersects the surface, the ramifications of chemical processing such as anodizing can develop the pores and create discontinuities in the AAO. See Figure 2.
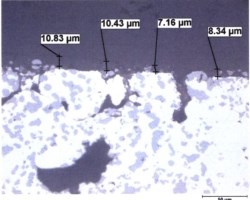
Figure 2: Alloy inhomogeneity coupled with surface connected porosity change the surface quality (chemical potential) and make it more difficult to anodize.
Surface Quality
Anodic oxide finish thickness variations after processing, even on components finished on the same rack can be caused by variations in substrate surface finish. Variation in these cases will not correspond to component location on the racks or to how the parts are contacted on the racks. Furthermore, thickness variation was also measured across the surface of individual parts, with the machined surfaces exhibiting a uniform and continuous finish thickness. Areas that aren’t machined (as-cast or shot peened or blasted) may exhibit discontinuous and unacceptably thin anodic oxide finishes.
As cast surfaces can be contaminated with residual mold release (parting compounds) or degradation/corrosion product from poorly maintained molds. Burrs, laps and the seams that they cause between the machined defect and the substrate are well documented sites of localized anodic charge concentration which prohibit uniform anodic oxide growth. These are points of resistance heating, hyper-growth and ultimately thin discontinuous anodic oxide finishes because, when the process current bias is imposed, the burrs stand immediately upright as the charged defects repel the surface (similarly charged objects repel one another). As burrs proceed to anodize through, breaking off into the electrolyte, they leave bare areas of substrate that begin to anodize later in the cycle. Because a blasted surface condition may not be uniform from component to component, the resultant finish thickness could never be uniform. See Figure no. 3.
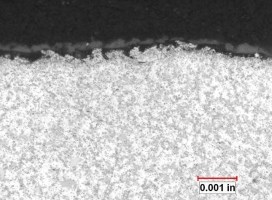
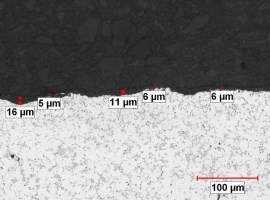
Figure no. 3: Documentary photograph of the shot peened surface of the die cast component before anodizing and after anodizing. The charge across the surface would be uniform during anodizing. The surface on the left is typical of a shot blasted surface. Note the non-uniformity of the surface. The charge distribution would be exceedingly non uniform, creating a situation where the finish growth would not be uniform, clearly documented by the nonuniform AAO finish thickness on the right.
Inasmuch as substrate consumption is part of anodizing, substrate defects introduced by way of mechanical processes will also be consumed, from as many surfaces as are exposed to the electrolyte. AAO formed around aluminum-based defects are prone to chipping and cracking. The tips of sharp corners and edges created by laps, folds or cracks introduced by finish machining and surface blasting are areas of charge concentration that result in localized high interfacial heat (increased reactivity) which may result in outgassing, burning and/or the formation of rounded voids in the coating. If the burr is anodized through its cross section, once detached from the substrate it will be included in the anodic oxide much like an inclusion. Non-aluminum abrasive media will not react with the electrolyte producing gaps in the anodic oxide or inclusion-like discontinuities.
Anodizing Complex Alloys
Anodic oxide formation is an electrochemical corrosion process that begins with nucleation at separate and distinct preferential sites across the substrate surface. Preferred sites are those which are not electrochemically complex, neither chemically (sites are comprised of aluminum only) nor topographically (surface is rather continuous with no burrs, laps or seams). In short, an ideal substrate is that which favors oxidation of aluminum.
In industry, an ideal substrate is rarely encountered. Products that are cast from aluminum alloys exhibit a variety of elemental additions that form alloy phases (which are homogeneous with the alloy system), intermetallic compounds (a compound of two metals that has a distinct chemical formula), precipitates (which fall out of alloy solution usually through heat treatment) and many other material defects that are on the microscopic and atomic level. Examples of atomic level defects are: grain boundaries, dislocations and vacancies, which are atomic level gaps and points of structural mismatch in the metallic crystal structure. The substrate electrochemical resistance changes with defects, alloy additions and contamination, especially when they intersect the surface, or interface between the substrate and the electrolyte. Therefore, all non-aluminum interfacial phenomena confound the anodizing reaction, retard it, and disrupt the anodic oxide structure.
Critical to understanding the side reactions that occur during anodizing is the recognition that the anodic oxide is an ionic solid comprised only of hydrated aluminum oxide. The structure exhibits minimal x-ray diffraction contrast, and is therefore designated amorphous but has been shown to exhibit short range order11, which gives it a pre-crystalline character. The anodic oxide structure is comprised only of aluminum and oxygen ions coordinated as AlO4 - tetrahedra which form the “network in the network” of self assembled, highly ordered nanoscale columns. The ionic solid facilitates ionic conduction which enables finish growth. Excess non-aluminum metal ions migrate through interstitial sites of the network lattice. Interstitial Al+3 ions migrate together with electrons from the electrolyte during anodizing. The electrolyte reacts with these sites at the surface of the of the pore walls.12 See Figure 4.
Next, it is critical to understand the aluminum substrate and the how alloying elements are present in the aluminum matrix. The aluminum substrate lattice is face centered cubic (fcc), which presents a structural mismatch between the substrate and the forming anodic oxide’s body-centered tetrahedral structure (bct). An aluminum alloy substrate lattice contains non-aluminum atoms in substitutional solution as well as interstitial intermetallic compounds that are not coherent with the microstructure. Since the anodic oxide will not comprise these non-aluminum atoms or intermetallic compounds, a compositional mismatch is also present between the substrate and the anodic oxide.
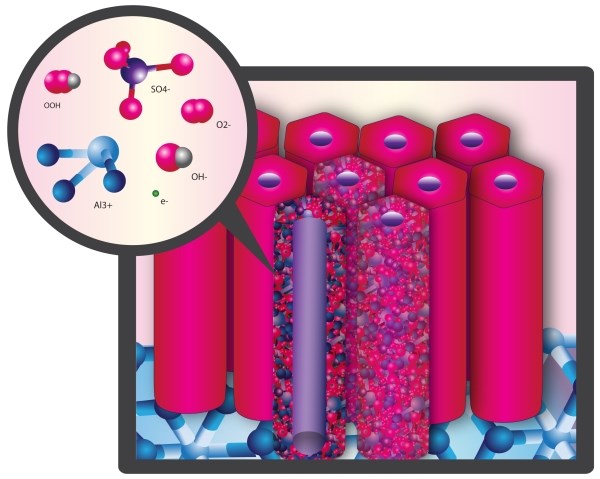
Figure 4: Representation of anodic oxide structure comprised of AlO4 tetrahedra with short range order. These tetrahedra comprise the internal network of the individual cells which make up the nanoscale network of the anodic oxide.
A thermodynamic mismatch is present because there are diffusion rate differences between metals and oxides (diffusion occurs faster in metals). Since the substrate is the source for the anodic oxide constituents, and the anodic oxide is the result of the continued reaction of the electrolyte with the substrate surface, the interface of the electrolyte and the surface is the key to continued anodic oxide formation and growth.
In nature, energy is required to overcome a mismatch. Energy produces heat. Non-aluminum metallic ions (copper, zinc, and other metal ions in solution with the aluminum alloy substrate) “pile up” at the interface and must realign themselves as they move from their positions on the substrate lattice to interstitial spaces within the anodic oxide lattice. Once they find a space, they move slower as they “hop” through the spaces in the anodic oxide network while aluminum ion diffusion occurs on the bct lattice which comprises the forming anodic oxide cell. The kinetics of the primary oxidation reaction are therefore retarded when anodizing complex alloys that are rich in non-aluminum elements in solid solution (such as in die castings). An increased resistance heating results at the interface; producing thin, rough finishes that often exhibit deposits of sooty-appearing reaction byproducts. See Figure 5.


Figure 5: Schematic of the walls of the anodic oxide at the junction of three anodic oxide cells. An entire cell is bracketed with pink dotted lines; the central pore appears gray with repulsive forces represented by a single sphere. The fcc aluminum structure is at the bottom of the schematic with the current bias represented by bold green arrows. The forming bct structure (A and B) exhibits the primary oxidation reaction at the interface between the fcc and the electrolyte in the pore by the presence of oxygen atoms on the bct structure. The schematic on the right B shows non-aluminum ions (copper colored) diffusing from the fcc lattice, through the bct anodic oxide network, into the central pore where it will be carried into the electrolyte. Note how the copper atoms “pile up” at the fcc-bct interface. This is due to the structural/compositional mismatch between the aluminum and the anodic oxide.
Inclusions, intermetallic compounds, precipitates, and other insoluble alloying elements that are incoherent with substrate microstructure are not anodized; instead, the primary oxidation reaction proceeds around them, taking them up into the anodic oxide. The impact of inert or insoluble defects such as inclusions and microconstituents that are not soluble in the aluminum matrix (e.g. lead and hypereutectic silicon) on the finish is in the spacing of the initial corrosion nuclei that precede the anodic oxide network and in the coherency of the AAO structure. This disruption in the order or the AAO structure can lead to irregular growth and irregular inter-column spacing. However, as the surface is consumed and a more ordered surface is presented for oxidation, the oxide growth recovers, but the surface of the oxide often replicates the defect, making the surface rougher. Mass transport of inclusions and insoluble species also occur through the network; although they are never incorporated into the structure itself. Side reactions such as these also retard the kinetics of the primary aluminum oxidation reaction.17 However, depending on the amount and distribution of incoherent defects, less energy (heat of reaction) is required at the interface to overcome such defects than the energy required to overcome the effects of substitutional elements in solution with the substrate on the anodic oxide formation and growth.
Complex alloys, such as die castings, offer mixtures of inclusions, phases and intermetallic compounds which confound the anodizing reaction. With as many various defects as the die casting contains, various side reactions occur to impede the surface reaction. The realignment of the non-aluminum substrate elements in solution and mass transport of inclusions and insoluble alloy additions such as hypereutectic silicon compete with the oxidation kinetics, resulting in repeated restructuring of the substrate surface which in turn disrupts the order of the anodic oxide. Depending upon the size and population of insoluble defects, the cohesive strength of the finish can be reduced and paths for environmental ingress can be created. This can reduce the corrosion resistance of the finish. Furthermore, the continuity of the porous structure will be interrupted, causing a marked decrease in dye uptake.
Overcoming the Barriers of Anodizing Complex Alloys: Recommended Process Parameters
The following recommendations are presented to aid in overcoming the barriers to anodizing die castings: 1) inspect and evaluate the components as received to make sure there are no obvious defects such as porosity or other discontinuities; 2) if the customer doesn’t want to pay for inspection, demand a premium cast product; 3) alter/improve/make the best of the surface with chemical cleaning and pretreatment; 4) optimize the anodizing process with proper electrolyte acid concentration, temperature and current density; and 5) consider further process optimization with a ramp or pulse anodizing.
As-cast components may exhibit residual dirt or mold release but will always exhibit an aluminum-rich surface due to the temperature gradient that occurs during solidification. Gentle soap cleaning may be all that is needed to prepare the surface for anodizing, provided all surface dirt is removed by cleaning. This may require frequent turnover of the cleaner solution.
Other chemical pretreatments are used to minimize the effects of non-aluminum alloy constituents that intersect the surface. In other words, these pretreatments are used to maximize the amount of free aluminum at the surface for anodizing. A note of caution: it is possible to overdo a good thing, as the pH of the cleaner (detergents, etchants, desmutters) typically indicates what in the alloy will be attacked/removed. Alkaline etches only remove aluminum, leaving behind non-aluminum residues such as copper which require a subsequent desmut step with Nitric acid or ferrous sulfate to remove. High silicon alloys sometimes require an acid etch (fluoride). Ammonium bifluoride etch solutions and Hydrofluoric acid/Nitric acid mixtures can be used to minimize the effects of silicon at the surface.
Because of the complexities of the chemical pretreatments and the variety of elements and surface dirt that they remove, it is extremely important to rinse well between treatment steps. Rinsing is the most important step in any anodizing operation.
Anodizing strategy should be developed around the alloy chemistry. Understand what alloying elements are in solution and what intermetallic compounds are present, and that everything ultimately intersects the surface. Work within moderate process parameters: medium range acid concentration, lower current density and use gentle ramping (turn up the bias/potential slowly to the desired current density/voltage). Interfacial resistance of the alloying elements is relieved by the careful imposition of anodizing parameter not by electrically blasting the surface.
Medium range acid concentration is recommended because higher acid concentrations corrode the component surface that has just been prepared by cleaning and pretreatment. While increased solution conductivity is an effective means to facilitate current flow through the electrolyte, it should not necessarily be viewed as a means to accelerate anodic oxide growth. In fact, the electrolyte can react with elemental copper, zinc or magnesium at the surface of the component, producing corrosion product barriers to the anodizing reaction. In order to initiate anodizing, a large input of electrical energy may be necessary to break through the corrosion product before a normal anodizing reaction can go forward at the surface. However, since surface corrosion product layers are typically discontinuous and nonuniform, anodic oxides with high roughness and perhaps thin or burned layers will form. Moderate acid concentration allows for a anodizing at a slightly reduced voltage for a given current density and always produces a smoother, more uniform finish.
Lower current density anodizing slows down the diffusion rate of electrons and metal ions in the component and the forming anodic oxide, giving the non-aluminum ions a chance to re-align themselves at the casting – AAO interface which in turn reduces resistance heating creating a smoother finish. By the same token, ramping and pulse anodizing help anodize complex alloys by giving non-aluminum ions in substrate solution time to diffuse, thereby relieving interfacial heat created by the competing reactions at the interface.
Summary
Review of several questions in Products Finishing Magazine regarding the anodizing of die castings illuminated four broad areas for discussion: alloy selection, substrate surface treatment, anodizing process parameters, and substrate quality. Through extensive literature research, scientific evaluation, both theoretical and through practical failure analysis, and, with practical experience, the conclusion follows that obtaining anodic finishes (anodized aluminum) of acceptable quality on die cast alloys is dependent primarily on the substrate quality of the die casting. These include the metallurgy of the substrate, alloy composition, casting quality, microstructure and surface quality. Substrate quality, therefore, is just as important, maybe more so, than anodizing conditions and technique.
Die castings are selected as the manufacturing process of choice usually because a number of complex components can be produced rapidly that will function near net shape. Alloys are first formulated such that they can be cast; next, that they yield the necessary mechanical properties for the application. Surface finish is often not very well considered, and although an anodic oxide finish may be desired to impart corrosion protection and wear resistance or to be a decorative finish, the ramifications of the anodizing process on a complex alloy is not typically considered at the design or foundry level.
Each factor has an impact on the interface from which the anodic oxide originates and grows13:
Alloy Selection: The elements which comprise the various cast aluminum alloys play a role in each of the three aspects of alloy selection: cast-ability, mechanical properties, and surface finishing. A basic understanding of the alloys, which elements are in solution (like copper, magnesium and zinc), which form intermetallic compounds, precipitates or fall out of solution as primary crystals (like silicon) is critical for developing effective approaches to finishing complex die cast components.
Surface Treatment: Interfacial defects are also introduced by external processes such as machining or residues from previous processes. Shot peening, grit blasting or other mechanical finishing techniques that are performed to clean and/or place the substrate in compression may leave residue that can also introduce interfacial contamination and will impede anodic oxide formation. Laps, seams and burrs created in the surface by these processes have distinct charge distribution effects on the substrate. Protrusions will exhibit charge concentrations and roots of substrate discontinuities will exhibit decreased reactivity due to repulsion forces set up by the proximity of like charges during the anodizing cycle.
Chemical pretreatments are element specific: Alkaline cleaners remove aluminum; acid cleaners “desmut” the surface, removing alloying elements exposed during alkaline cleaning. Acid-based fluoride etches or buffered bifluoride formulations act to remove silicon. All steps are intended to yield as much free aluminum, and as uniform charge distribution at the surface for anodizing as possible.
Anodizing Parameters: It is commonly viewed that increased solution conductivity (reducing the solution resistance) will aid in successful anodizing of complex alloys. This is not to be confused with the coil anodizing process in which increased solution conductivity is necessary to match the speed with which the coil is exposed to the electrolyte. Maximum solution conductivity has been measured at 375 g/l for sulfuric acid electrolytes14 and successful anodizing is routinely carried out at concentrations much less than that of maximum conductivity, typically 180 g/l to 220 g/l.
With die cast aluminum alloys with elements such as copper in solution (phases, not intermetallic compounds or inclusions), it is best to recognize that time is required for diffusion of the non-aluminum constituent(s) through the hydrated aluminum oxide ionic solid as it forms. This is achieved by beginning the process with a slow ramp to the appropriate anodizing voltage, if anodizing potentiostatically; or to the appropriate current density, if anodizing galvanostatically. It has been shown that some complex alloys can be anodized to sufficient thickness if anodized in electrolytes of typical concentration, at intermediate to high current densities (1.8 – 3.2 amps/dm2 [16 – 30 amps/ft2]) at intermediate or even room temperature, without compromising significant hardness.
Substrate Quality: When the anodizing process is under control, if things go wrong with finishing a component, it is reasonable to conclude that the source for the problem is not the anodizing. It is important to know how to proceed to troubleshoot effectively. Understanding component failures from the point of view that anodizing develops the surface of the part is important because metal finishing is the last step of the manufacturing process. Therefore, if something unfavorable develops, all eyes will be on the metal finisher. At times like these, substrate quality needs to be metallurgically evaluated. A metallurgical engineer need not be employed by the metal finishing plant; however, it is essential to know when one is needed to investigate a root cause for failure.
Conclusion
There is no “trick” to anodizing complex alloys; there are ways, grounded in scientific reason, to approach the challenges.
- Start with as homogeneous a substrate surface as possible. This means a clean substrate, free of oil and dirt, without casting defects such as porosity intersecting the surface, or laps, burrs or seams created by machining, peening or blasting of the surface.
- As much free aluminum should be present at the surface as possible. This can mean after cleaning, that the surface is alkaline treated to remove a small amount of aluminum at the surface in order to expose alloying elements such as copper and silicon. These elements can be removed through a nitric acid desmut operation (to remove the copper) and/or a treatment to remove the silicon such as a dilute hydrofluoric acid etch or an ammonium bifluoride treatment.
- After a good rinse to prevent dragging these undesired elements into the anodizing electrolyte, or leaving them to redeposit on the component surface, anodize in an electrolyte whose acid concentration is efficient enough to carry the current without corroding the surface. Depending upon the alloy complexity, this is typically from 180 to 220 grams/liter H2SO4. The current bias should be applied gently, via a ramp or with intermittant pulses, because time is required to allow for the diffusion of the non-aluminum elements during anodized aluminum oxide (AAO) growth. A slower AAO growth will always lead to a more uniform anodized finish.
- It may be desired to follow finishing with a dilute HNO3 rinse to remove any interstitial copper from the AAO surface, to enable dye uptake and to prevent isolated spots of copper corrosion product from the surface.
- Rinsing is imperative between process steps to keep alloying elements from building up in the process tanks.
It is also important to know and understand the component history should anodizing problems arise to connect manufacturing variations to variations in anodic oxide appearance or function. Such understanding enables best possible alloy selection for manufacturing and metal finishing; appropriate adjustments to the anodizing process, or target areas of the casting/manufacturing processes and/or the metal finishing process for corrective action.
Acknowledgement
The authors would like to acknowledge Ms. Joy Kaufman of Joyjoycreations, Inc. for her illustrations that help to explain the diffusion and structural differences that occur during anodic oxide formation.
References
1 – 8. Chesterfield, Larry. 2001 – 2009 Aluminum Anodizing Clinic, Products Finishing, Vol. 65 No. 11 - Vol. 74 No. 2
9. North American Die Casting Association, www.diecasting.org, FAQ, Introduction.
10. Davis, J. R., editor, ASM Specialty Handbook, Aluminum and Aluminum Alloys, ASM International, Materials Park, Ohio, 1993.
11. Brodalla, Dieter, “Struktur und Molekulares Design Anodisch Erzeugter Aluminiumoxidschichten und der Mechanismus der Verdichtung“, reprint received 2007.
12. Runge, J., “Formation of Porous Anodic Oxide Finishes – A New Approach and Theory”, Proceedings, Aluminium 2000, 2007.
13. Runge, J., “Interfacial Phenomena and Anodizing: Ramifications and Process Solutions”, Proceedings of the 17th Annual AAC Conference, San Francisco, California, 2008.
14. Brace, A., The Technology of Anodizing Aluminium, 3rd Edition, Interall Srl, 2000.
Related Content
NADCAP Shop Digitizes to Eliminate Paper Trail
Customizable ERP software has transformed a 27-year-old manual metal finishing job shop into a state-of-the-art paperless company with full digital traceability in about 10 months.
Read MoreBryan Leiker, MFACA, Discusses CARB Public Hearing Over Calif. Hex Chrome Ban
Bryan Leiker, executive director, Metal Finishing Association of California, offers a recap of a January 27, 2023, public hearing conducted by the California Air Resources Board prior to an impending ruling on a proposed ban of hexavalent chromium use for finishing operations in the state.
Read MoreUnderstanding and Managing White Spots on Anodized Aluminum
Having trouble with spotting defects when anodizing? Taj Patel of Techevon LLC offers a helpful overview of the various causes of white spots and potential solutions.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More













.jpg;maxWidth=300;quality=90)








