A Process for Alkaline Non-cyanide Silver Plating for Direct Plating on Copper, Copper Alloys and Nickel Without a Silver Strike Bath
Traditionally, silver is electroplated in toxic, cyanide-based chemistry. Due to cyanide’s extreme hazard to human health and environments, developing non-cyanide silver chemistry is essential for the silver electroplating industry. Discussed here is an aqueous, alkaline non-cyanide silver plating technology, which can be directly plated over nickel as well as copper and its alloys. The silver deposits have perfect white color and better anti-tarnishing properties than other non-cyanide silver processes. The silver is plated entirely from the dissolving silver anode and the bath is very stable, and maintains a stable pH level both during plating and idle time. This new non-cyanide silver technology will plate bright silver that is perfectly suitable for electronic, industrial and decorative applications. .
by Eric Olander* and John Cheng
EPI, Electrochemical Products Inc.
New Berlin, Wisconsin, USA
Editor’s Note: This paper was presented on November 2, 2021, at SUR/FIN 2021 in Detroit, Michigan, in Session 4: Advances in Surface Finishing Technology. A printable pdf of this paper can be accessed and printed HERE, and the accompanying Powerpoint presentation can be accessed HERE.
ABSTRACT
Silver electroplating is one of the most important plating processes for decorative and electronic applications. Traditionally, silver is electroplated in toxic, cyanide-based chemistry. Due to cyanide’s extreme hazard to human health and environments, developing non-cyanide silver chemistry is essential for the silver electroplating industry. Here, we present our advanced proprietary aqueous, alkaline non-cyanide silver plating technology**, which can be directly plated over nickel as well as copper and its alloys. The silver deposits have perfect white color and better anti-tarnishing properties than other non-cyanide silver processes. The new chemistry is very cost-effective, as the silver is plated entirely from the dissolving silver anode. The bath is very stable, the pH is very well buffered and maintains a stable pH level both during plating and idle time. This new non-cyanide silver technology will plate bright silver that is perfectly suitable for electronic, industrial and decorative applications.
Introduction
Silver electroplating has been traditionally used for decorative applications such as silverware. Because of its excellent electric characteristics, silver plating has been widely used in electronic industries for switches, connectors and the like.
Ever since the first patent in 1840, silver electroplating has usually been plated in cyanide baths, which contain the most toxic chemical (cyanide) and carries an extremely high risk to human health and to the environment. Therefore, tremendous efforts have been attempted to develop cyanide-free electroplating systems with excellent stability and environmental friendliness.
When designing aqueous alkaline non-cyanide silver plating chemistry, the following aspects have to be considered:
- Environmental friendliness: the complex used to chelate silver should be environmentally friendly. The silver ions can also be readily precipitated to be reclaimed or waste-treated;
- Stable bath chemistry: stable under lights, no change of pH;
- Plates over different metal substrates, especially over nickel;
- Excellent adhesion between silver and metal substrates: no immersion silver on metal substrates or the silver immersion layer has no detrimental effect on adhesion and
- White silver deposit with no yellow hues: excellent anti-tarnishing properties, etc.
Here we present our new alkaline non-cyanide technology, which can plate non-cyanide silver directly on nickel surfaces (meeting all three types of ASTM B-700), as well as silver, brass, bronze and copper, and does not require a separate silver strike on these substrates (Fig. 1[A,B]). The plating bath is an alkaline, cyanide-free plating solution, which can plate bright silver for electronic, industrial and decorative uses. It operates at room temperature, can be utilized in both rack and barrel plating and provides extremely stable alkaline non-cyanide silver plating chemistry. The silver deposit has exceptional covering and throwing power. The plating chemistry produces a fine-grained, smooth, dense, silver deposit with low porosity and excellent bonding properties. The silver deposit exhibits a superior, brilliant white color and shows improved anti-tarnishing properties (Fig. 1C). This new chemistry is cost-effective because it plates entirely from the corrosion of silver anodes rather than salts in solution. Maintaining the chemistry is easy with a maintenance electrolyte additive and a brightener and/or grain refiner.
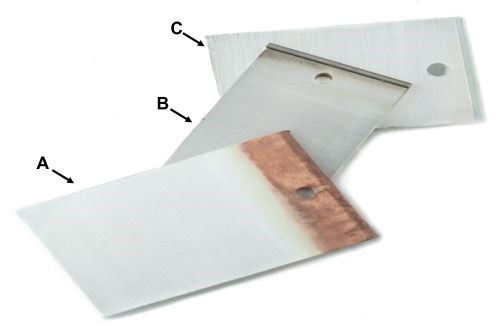
Non-cyanide alkaline silver characteristics: (A) silver on copper without Ag strike; (B) silver on nickel without Ag strike; (C) silver deposit appearance.
Plating bath setup and bath specification
The bath is an alkaline, cyanide free liquid solution, which contains silver ion, maintenance electrolyte, brightener and DI water. The bath is a clear solution. The pH is maintained between 9.5 and 10.5. If the pH is below 9.5, one can adjust with KOH. If the pH is over 10.5, 50% nitric acid is used for adjustment. It is critical that the pH of the bath not exceed 11.5. The plating specifications for rack and barrel baths are as given in Table 1.
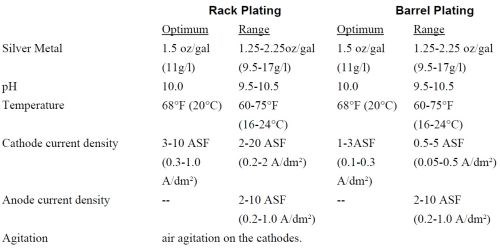
Table 1 - Plating specifications.
Note that the silver metal concentration in both the rack and barrel formulations are about 30% of the concentration found in most cyanide baths. Thus, the silver drag-out losses are greatly reduced with this non-cyanide bath.
Operation of the plating bath
Plating rate
After the initial make up, the silver bath must be filtered continuously using micron or smaller spun-polypropylene cartridge. The optimum cathode current density for this non-cyanide silver bath is 3-10 ASF. The cathode efficiency is around 90-95%. Table 2 shows the plating rate for various current densities.

Table 2 - Plating rate versus cathode current density.
Maintenance
The plating bath consists of:
- Liquid silver concentrate for silver metal replenishing, which is only used for initial bath make-up;
- Liquid electrolyte concentrates for electrolyte replenishing. Periodic adds over time are based on ampere-hours, Hull Cell tests or analysis by HPLC (Fig. 2) and
- Liquid concentrate of brightener and grain refiner. Periodic adds over time are based on ampere-hours, Hull Cell tests or analysis.
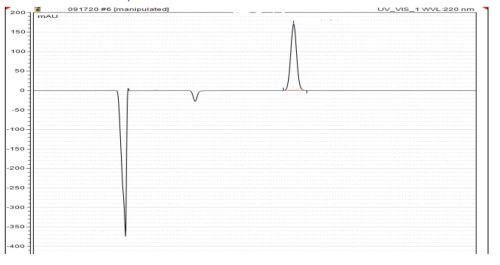
Figure 2 - HPLC results.
The pH of the solution is buffered and very stable during plating and storage. Silver is supplied by the slab silver anode or popcorn anodes in a polypropylene basket. It is very cost-effective because it plates out of the silver anodes rather than the solution.
The silver-stabilizer and its breakdown products can be analyzed by high-performance liquid chromatography (HPLC)(Fig. 2). Through industrial practice, this non-cyanide silver process shows that the breakdown products have no ill-effect on the physical properties of silver deposit.
The solution must be kept free of suspended matter in order to prevent roughness. Continuous filtration with a one-micron filter is recommended. A new filter cartridge must be flushed prior to use by circulating DI water through it. A high volume, low pressure, air source is required for air agitation of plated parts to avoid burning and produce better silver qualities.
Physical properties of non-cyanide silver deposit
Appearance
Initially, the parts had a slightly dull appearance, which was quickly converted to a bright white by dipping them momentarily in dilute sulfuric acid. The throwing was excellent. The bath did not develop an immersion coating on copper and nickel and excellent adhesion can be achieved without needing a strike bath. The SEM morphology of normally operated silver deposit is shown in Fig. 3. The deposit is smooth and has a fine grain structure. The crystal structure of the silver coating is also characterized by X-ray diffraction. The broader diffraction peak of a non-cyanide deposit suggests a finer grain than that of the cyanide silver deposit.
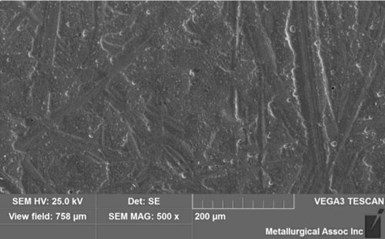
Figure 3 - Silver deposit morphology by SEM.
Hardness
The hardness of non-cyanide silver coating is around 150-200 KHN50, which is slightly harder than the cyanide silver from solution with no additives, and from a first generation, non-cyanide silver deposit*** which had a hardness of 95-110 KHN25 (indenter at a load of 25 grams). The hardness was measured on a cross section sample with a Knoop diamond indenter at a load of 50 grams.
Electrical resistance
The electrical resistivity of the non-cyanide deposit is around 3.0-3.5 microhm-cm, which is slightly higher than that of the cyanide silver deposit and is suitable for electrical applications.
Wear resistance
The wear resistivity of the non-cyanide silver deposit is superior to that of the cyanide silver deposit. The Taber abrader wear test abraded the silver deposit with a load of 250 grams.
Purity
The purity of the non-cyanide silver deposit is over 99.9%, as analyzed and certified by an outside accredited lab.
Conclusion
A novel, breakthrough non-cyanide electroplating chemistry has been developed. The silver deposit has a brilliant white color and shows improved anti-tarnishing properties compared to other non-cyanide silver processes. The new chemistry is very cost-effective, and it plates entirely out of silver anodes. The bath is extremely stable. The solution pH is buffered and remains stable during plating and when idle. It can plate non-cyanide silver directly on nickel surfaces (meeting all three types of ASTM B-700) as well as plate directly on silver, brass, bronze and copper and does not require a separate silver strike on these substrates. The plating bath is an alkaline, cyanide-free plating solution, which can plate bright silver for electronic, industrial and decorative uses.
About the authors

Eric Olander is Owner and President of EPi - Electrochemical Products Inc., of New Berlin, Wisconsin. He received a B.S. in Chemical Engineering from Iowa State University. He is a Past President of the AESF Milwaukee Branch AESF and a past member of the MFSA Board of Trustees. Mr. Olander has served of the SUR/FIN Steering Committee and the MFSA Membership Committee. He was the recipient of the 2010 August P. Munning Award and was named an NASF Fellow in 2018.
John Cheng is R&D Manager as well as heading up Asian Market Development efforts.
* Corresponding author:
Eric Olander CEF
Owner / President
EPI
17000 W. Lincoln Ave.
New Berlin, WI 53151 USA
Phone: (262) 786-9330
E-mail: erico@epi.com
** E-Brite® 2.0 Ag, Electrochemical Products Inc., 17000 W. Lincoln Ave., New Berlin, WI 53151.
*** E-Brite® 50/50, Electrochemical Products Inc., 17000 W. Lincoln Ave., New Berlin, WI 53151.
Related Content
MicroCare Appoints Commercial Sales Manager
MicroCare welcomes Kristin Rosas as its strategic account executive for its Precision and Medical Device Manufacturing Fluids divisions.
Read MoreCustom Plating Technology Enables Medical Devices
Finisher collaboration during product design delivers plating solutions for medical OEMs.
Read MoreSilica-free Abrasive Compounds Offer Improved Health, Safety
Osborn has a line of silica-free abrasive compounds that offer both employee and environmental benefits.
Read MoreElectroplating for Medical Devices
Brittany McKinney of Pavco Inc. discusses the benefits of electroplating for medical devices.
Read MoreRead Next
Episode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More





















