How Dissolved Aluminum in Low Caustic Etch Baths Affects Acid-Etched Surfaces
Decreasing the concentration of aluminum in the low caustic step can also lead to problems such as preferential etching.
Aluminum and its alloys have been widely used over the past many decades, especially in the aircraft industry, due to its excellent weight-to-strength ratio and corrosion resistance. The need to reduce weight-to-strength ratios makes it particularly attractive to the building industry, but it also is being used increasingly in the automotive industry, along with defense, ship construction, packaging, building and others.1
Aluminum cannot be used as produced, however, due to its amphoteric nature, by which the native oxide film on the aluminum surface tends to corrode under alkaline and/or acidic environments. It therefore has to first undergo surface treatment processes such as painting, lacquering or anodizing. Among those processes, anodizing provides the longest surface life with higher chemical abrasion resistance while maintaining the metallic texture of the surface.1,2 The building industry in particular requires these properties. But unlike with lacquering or painting, anodizing has a tendency to enhance any defects on the surface originated from the base metal. These defects are preferentially etched in the pretreatment step as a result of the higher chemical potential, and they resurface even stronger before anodizing is applied.2 The problems caused by the base metal, such as grain growth, segregation and streak marks, originate mainly from the production parameters (extrusion speed, die shape, cooling speed and even heat-treatment conditions) in the prior steps to anodizing.3,5
The need for more aluminum profiles with the desired esthetic properties has increased the demand for a replacement to conventional long-life caustic etching processes, the main cause of preferential etching and non-uniform surface appearance. Acid etch processes have been developed which create an even surface that masks these surface defects, however, the post-treatment caustic step (called the “low caustic” step) necessary to remove the aluminum fluoride residue from the surface can also lead to preferential etching and require resurfacing due to the lack of dissolved aluminum to inhibit the aggressiveness of the caustic.1-3
Increasing the dissolved aluminum amount would be the ideal option, but this solution can have two main drawbacks: increasing the process time and increasing the viscosity of the bath. Although the process time can be shortened by raising the temperature, elevated viscosity can cause many other problems, including increased dragout, uneven etching, “tiger stripes” and problems in the desmuting stage.2,6
The increase in demand for aluminum is correspondingly pushing the capacity of existing facilities, inclining them to sacrifice some of the surface properties required for anodizing. Profiles with relatively poorer attributes require more masking than standard profiles, therefore products with complex shapes and increased extrusion speed are treated with acid etching prior to anodizing. But nowadays even the concealing capability of acid etching can fail to meet the standards, due to the process parameters of the low caustic post-treatment.4,7
Experimentation
Here, we describe experiments targeting the process parameters of the low caustic bath after the acid etching, attempting to maximize the defect-hiding capability of the acid etch process. Two main sets of samples were prepared and treated in low caustic baths with different dissolved aluminum concentrations at various process parameters. The first set of tests studied the effect of dissolved aluminum on the aluminum surface; only the amount of dissolved aluminum was changed and all the other parameters of the acid etch and low caustic step were the same. In the second set of tests, the surface gloss of the samples was adjusted to relatively similar values to study the effect of dissolved aluminum on the defect-concealing property of the acid etch process. The surface gloss was adjusted by changing the process time in various low caustic baths with different levels of dissolved aluminum. By comparing the second set samples using SEM and 3D profiling analyses, we were able to obtain the best possible flaw-masking properties for the combination of acid etch and low caustic processes.
The study used 6061 aluminum profiles. The chemical composition of the aluminum was investigated by using optical emission spectroscopy, and the results are shown in Table 1.
![Table 1: Chemical composition of the samples (wt%) Results Fe Si Cr Mn Mg Zn Cu Ti Others Al [wt%] 0.5 0.6 0.1 0.8 1.2 0.2 0.8 0.1 0.15 Rest](https://d2n4wb9orp1vta.cloudfront.net/cms/brand/PF/2018-PF/09September/t1.jpg;maxWidth=600)
Before the etching process, the specimens were degreased by dipping in a 45 g/l Alumal Clean 115 solution. The first set was etched at 40°C for 4 minutes in an acidic etch bath followed by the low caustic etch process. To investigate the effect of dissolved aluminum on the surface morphology, various amount of dissolved aluminum containing solutions were added. The caustic baths contained 60 g/l sodium hydroxide (NaOH), 35 g/l Alumal Etch LMA 235, and 10, 50 or 100 g/l of dissolved aluminum. The etching time was 2 minutes for post-treatment at 55°C (Samples A,B and C). Additionally, samples were pre-treated using conventional alkaline etch and acidic etch parameters as control samples for comparison (Samples D and E). The treatment parameters and code numbers of the samples are shown in Table 2. The surface gloss of the samples was measured using a Novo-Gloss Lite glossmeter.
![Table 2: Acid Etch and “Low Caustic” Process Conditions for the First Set of Samples Acid Etch Temp. [°C] Time [min] 40 4 40 4 40 4 45 4 Caustic Etch Temp. [°C] Al Cons. [g/L] Time [min] 55 65 10 2 50 2 100 2 100 0.25 100 12 Sample Code A B C D E](https://d2n4wb9orp1vta.cloudfront.net/cms/brand/PF/2018-PF/09September/t2.jpg;maxWidth=600)
In the second phase of the study, new specimens with the same surface gloss were produced by using different low caustic baths at various dissolved aluminum concentrations. The gloss of the samples was adjusted by controlling the process time to enable a relevant comparison of the masking properties after being subjected to a similar etching (see Table 3).
![Table 3: Acid Etch and “Low Caustic” Process Conditions for the Second Set of Samples Acid Etch Temp. [°C] Time [min] 40 4 Caustic Etch Temp. [°C] Al Cons. [g/L] Time [min] 55 10 100 0.5 1 1.5 2.5 3 3.5 4 Sample Code A2 A3 A4 C2 C3 C4 C5](https://d2n4wb9orp1vta.cloudfront.net/cms/brand/PF/2018-PF/09September/t3.jpg;maxWidth=600)
The surface morphology of the samples was characterized by using SEM/SEI (JEOL JSM_7000F), 3D surface profiling (WYKO NT1100 Veec) and a glossmeter.
Results
The R values and the three-dimensional surface topographies of the first set samples (A-E) were investigated using 3D profilometry. The results are presented in Table 4 and Figure 1.
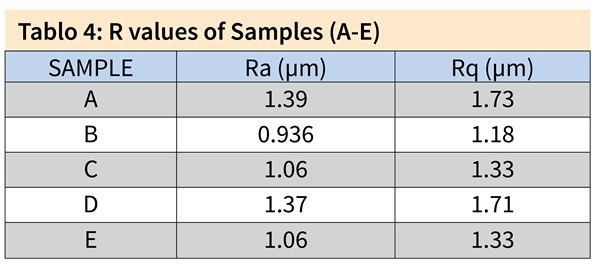
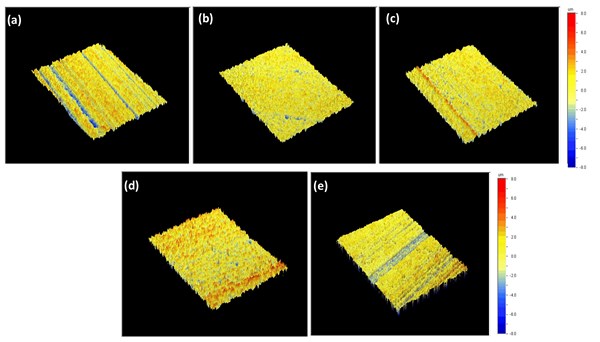
Figure 1. 3D topologies of the first set of samples, Samples A, B and C all pretreated in acid etch bath at 40°C for 4 minutes and post-treated in a low caustic bath containing 60 g/l NaOH. (a) Sample A included 10 g/l dissolved Al for 2 minutes at 55°C, (b) Sample B 50 g/l dissolved Al for 2 minutes at 55°C and (c) Sample C 100 g/l dissolved Al for 2 minutes at 55°C. (d) Sample D was pretreated in acid etch bath at 45°C for 4 minutes and post-treated in a low caustic bath containing 60 g/l NaOH and 100 g/l dissolved Al for 15 seconds at 55°C and (e) Sample E in 100 g/l dissolved Al at 40°C for 12 minutes.
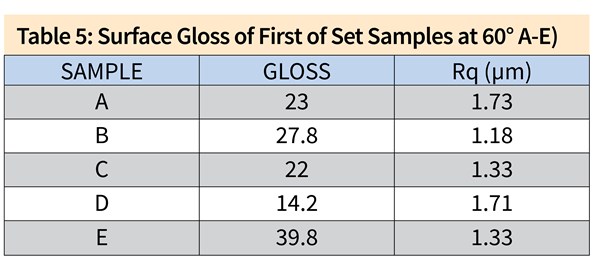
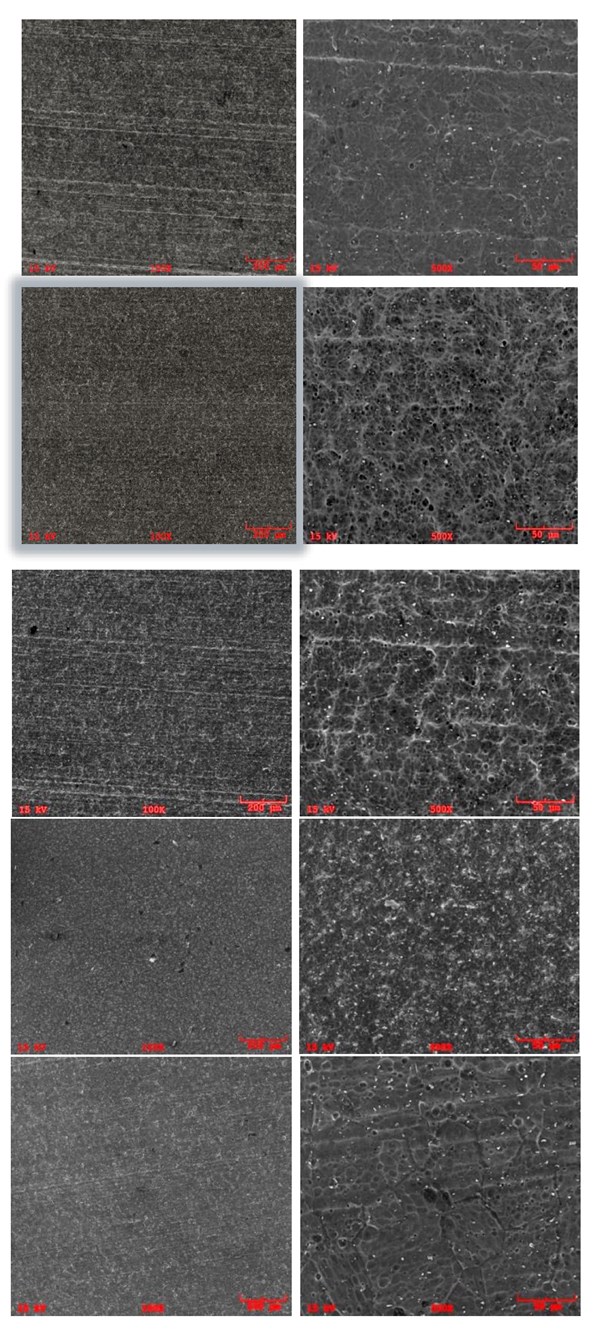
\Figure 2. SEI/SEM images of the first set of samples. (a) Sample A, 100X, (b) Sample A, 500X, (c) Sample B, 100X, (d) Sample B, 500X, (e) Sample C, 100X, (f) Sample C, 500X, (g) Sample D, 100X, (h) Sample D, 500X, (i) ) Sample E, 100X, (j) Sample E, 500X.
Figure 2 shows the SEM analyses on the first set of samples. The results are in accordance with the findings from 3D profiling, in which Samples B and C show similar surface texture to Sample D (acid etch control sample): pits with sharp edges distributed throughout the surface. The surface of Sample A resembles that of Sample E (long-life caustic etch control sample), which has smooth-edged alkaline pits with intermetallics reemerging from the aluminum matrix. On Samples A and E, due to the abrasive nature of the caustic etching step, even the grain boundaries were noticeable because of the preferential etching, which decreased the uniform appearance.
Table 6 shows the surface gloss values achieved by changing the process times in the low caustic step for Samples A, B and C. From these results, we can see the hindering influence of dissolved aluminum. The same gloss values were achieved for low-aluminum-content Sample A3 in 1 minute and for high-aluminum-content Sample C4 in 3.5 minutes. Samples A3, B and C4 were chosen for further investigation due to their relevant surface gloss values.
Table 7 and Figure 3 show the 3D profiling results alongside the surface roughness values, which indicate a similar surface topology. The major difference in the results appears to be between Samples A and C, where Sample A exhibits sharper peaks due to the shorter process time in low caustic, decreasing the uniform surface topology. Sample C shows the highest surface roughness and highest difference in high and low points due to the extended time in the low caustic step, thus causing the surface defects to reemerge and limiting the masking effect of the acid etch process.
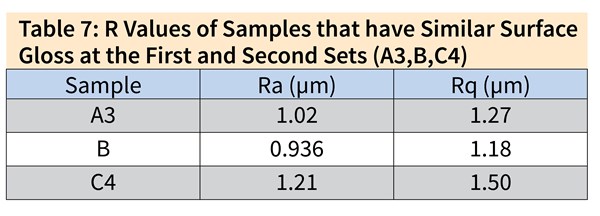
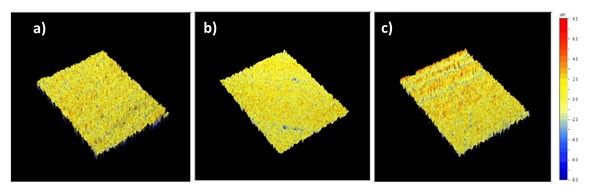
Figure 3. 3D topologies of the second set of samples, all pretreated in acid etch bath at 40°C for 4 minutes and post-treated in a low caustic bath containing 60 g/l NaOH and (a) Sample A3 with 100 g/l dissolved Al at 40°C for 12 minutes and 10 g/l dissolved Al for 1 minute at 55°C, (b) Sample B, 50 g/l dissolved Al at 40°C for 2 minutes at 55°C and (c) Sample C4, 100 g/l dissolved Al for 3.5 minutes at 55°C.
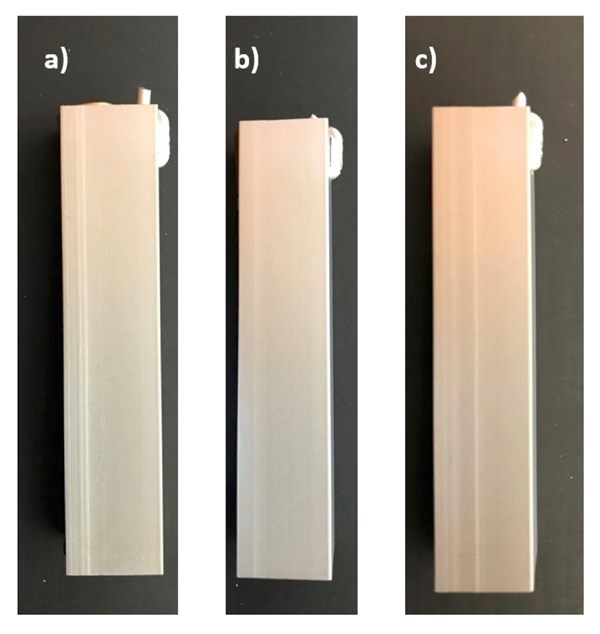
Figure 4. Macro images of second set samples. (a) Sample A3, (b) Sample B and (c) Sample C4
Figure 5 illustrates the SEM analyses of Samples A3, B and C4, showing the surface morphology after the acid and low caustic etching steps are completed. The results show that Samples B and C4 exhibit similarity in the shape and distribution of the pores, with smoother sides and less intermetallics. On the other hand, Sample A3 has pits with sharper edges, and the intermetallics are still detectable, caused by the shorter time in low caustic step.
Combining this data with the findings from the first set of samples and Figure 4, it is safe to say that the amount of dissolved aluminum in a low caustic bath has a determining effect on the final morphology of the surface. To obtain the maximum amount of defect masking effect from the acid etch process, the aluminum concentration in the low caustic bath has to be carefully adjusted, since the lack of dissolved aluminum can cause over-etching and thus resurfacing of the defects, or the excess amount of dissolved aluminum can lead to uneven etching as a result of the elevated time in the bath.
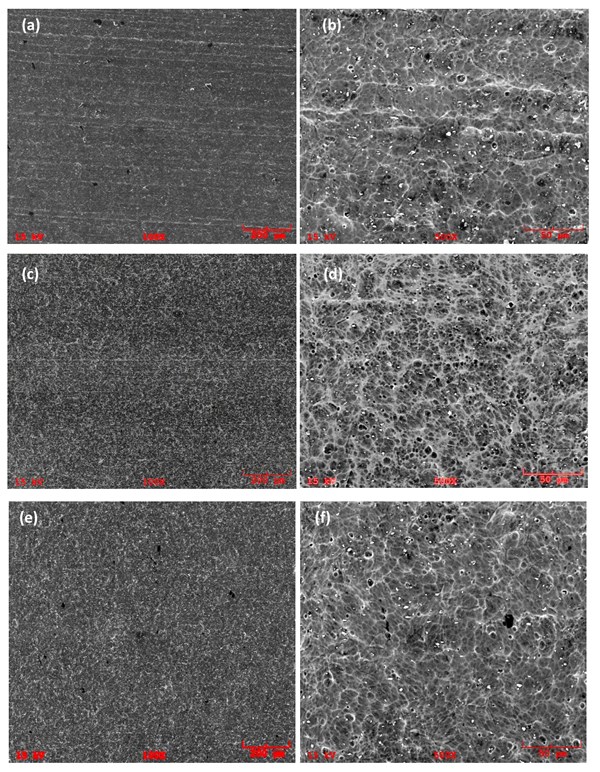
Figure 5: SEI/SEM images of second set samples; (a) Sample A3, Pre-treated in acid etch bath at 40 oC for 4min and post treated in “low caustic” containing 60 g/l NaOH and 100 g/l dissolved Al at 40 oC for 12min 10 g/l dissolved Al for 1min at 55 oC 100X , (b) Sample A3, Pre-treated in acid etch bath at 40 oC for 4min and post treated in “low caustic” containing 60 g/l NaOH and 100 g/l dissolved Al at 40 oC for 12min 10 g/l dissolved Al for 1min at 55 oC, 500X (c) Sample B, Pretreated in acid etch bath at 40 oC for 4min and post treated in “low caustic” bath containing 60 g/l NaOH and 50 g/l dissolved Al at 40 oC for 2min at 55 oC, 100X, (d) Sample B, Pretreated in acid etch bath at 40 oC for 4min and post treated in “low caustic” bath containing 60 g/l NaOH and 50 g/l dissolved Al at 40 oC for 2min at 55 oC, 500X, (e) Sample C4, Pretreated in acid etch bath at 40 oC for 4min and post treated in “low caustic” bath 100 g/l dissolved Al for 3.5min at 55 oC, 100X, (f) Sample C4, Pretreated in acid etch bath at 40 oC for 4min and post treated in “low caustic” bath 100 g/l dissolved Al for 3.5min at 55 oC, 500X
Conclusion
The results of these experments indicate that decreasing the concentration of aluminum in a low caustic step can also lead to problems such as preferential etching, causing reemerging of surface defects or over-etching, which results in non-uniform surface appearance.
On the other hand, the concentration of dissolved aluminum in the low caustic bath can increase the beneficial effect of the acid etch process to disguise the defects originating from the raw material. The data has clearly shown that, above 50 g/l concentration, the beneficial effect of dissolved aluminum also starts to decrease as a result of the extended time in the low caustic bath. Furthermore, during full-scale production, increasing the aluminum concentration in the bath will also increase the viscosity of the bath, thus inhibiting another advantage of the acid etch process, which is lesser dragout and lesser caustic residue for complex shaped profiles.
Can Akyil, Pınar Afsin, Mesut Akkaya and Mustafa Urgen are with Politeknik Metal San. Tic. A.S., a member of Coventya Group. Visit coventya.com.
References
1Sheasby, P.G., Pinner, R. (eds), (2001), The Surface Treatment and Finishing of Aluminium and Its Alloys, Volume 1, The Fundementals of Anodizing, (pp. 112-358), ASM International Finishing Publications Ltd., 2001.
2Sheasby, P.G., Pinner, R. (eds), (2001), The Surface Treatment and Finishing of Aluminium and Its Alloys, Volume 1, The Fundamentals of Anodizing, (pp. 393-488), ASM International Finishing Publications Ltd., 2001.
3D. Kanagaraj, V.L. Narasihmhan, S.V. Iyer, Anodizing of Aluminum in Sulphamic Acid Electrolyte, Bulletin of Electrochemistry 12 (5-6) May-June 1996, pp. 288-290.
4Jinsub Choi, Fabrication of Monodomain Porous Alumina Using Nanoimprint Lithography And Its Applications, Ph D. Thesis, Mathematisch-Naturwissenschaftlich-Technischen Fakultat (Ingenieurwissenschaftlicher Bereich) der Martin-Luther-Universitat Halle-Wittenberg 2003, pp. 2-12.
5Hunter, F., Hohn, P. (1992) Caustıc Etchıng On Alumınum Wıth Matte Finish And Low Waste Other Publıcatıons Capabılıty, US Patent, No: 5091046, dated Feb. 25, 1992.
6Davis J.R., 1993 Fabrication and Finishing of Aluminum Alloys, ASM Specialty Handbook: Aluminum and Aluminum Alloys, pp. 460-463. ASM International.
7Martin, T., Hebert, K.R., (2001), Atomic Force Microscopy Study of Anodic Etching of Aluminum - Etching Morphology Development and Caustic Pretreatment, Journal Of The Electrochemıcal Socıety Volume: 148 Issue: B101-B109.
Related Content
How to Choose Between Sulfate and Chloride-Based Trivalent Chromium
There are several factors to consider when choosing between sulfate and chloride-based baths for trivalent chromium plating. Mark Schario of Columbia Chemical discusses the differences and what platers should keep in mind when evaluating options.
Read MoreInnovation in Plating on Plastic
Plating on advanced plastics solution offers improved adhesion, temperature resistance and cost savings.
Read MoreProducts Finishing Reveals 2024 Qualifying Top Shops
PF reveals the qualifying shops in its annual Top Shops Benchmarking Survey — a program designed to offer shops insights into their overall performance in the industry.
Read MorePossibilities From Electroplating 3D Printed Plastic Parts
Adding layers of nickel or copper to 3D printed polymer can impart desired properties such as electrical conductivity, EMI shielding, abrasion resistance and improved strength — approaching and even exceeding 3D printed metal, according to RePliForm.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreMasking Solutions for Medical Applications
According to Custom Fabricating and Supplies, a cleanroom is ideal for converting, die cutting, laminating, slitting, packaging and assembly of medical-grade products.
Read More





















