Overview: Aerospace Anodize Finishes
The following anodizing process overviews are provided as a means of introduction to aerospace anodizing
Share
Written by Gary Kriesch of the Walgren Co., Warren John Fullen of Boeing and Earl Turns of Bell Helicopter, with a major contribution from Julie Unangs of Boeing.
Presented at the 19th Annual International Anodizing Conference & Exposition, October 5-7, 2010, Montreal, Quebec, Canada. For more information, please visit Anodizing.org
The following anodizing process overviews are provided as a means of introduction to aerospace anodizing. These overviews are not meant to be used as a processing guide. The applicable process specifications provide the full complement of process requirements.These process specifications are typically formatted as follows:
- Scope
- References
- Materials Control
- Facilities Control
- Definitions
- Manufacturing Control
- Maintenance Control
- Quality Control
- Test Methods
- Qualification
Phosphoric Acid Anodizing
A common likely first step in a bond failure is hydration of the aluminum oxide layer. Phosphoric Acid Anodizing (PAA) was developed to improve bond reliability for “metal-bond” structure parts. The PAA process was established in the mid 1970’s as a reliable production process (Ref. 1). Furthermore, the hydration resistant oxides of PAA result in environmentally durable bonded part components.
The prescribed surface preparation and anodize process provides a method for producing and controlling the unique oxide characteristics. Robust processing is evident via the relatively wide operating process ranges and the known long bath life of PAA.
Chromic Acid Anodizing
Chromic acid anodizing (CAA) is used for forming coatings on structural parts. CAA coatings have little or no effect on the fatigue strength of finished parts because they are very thin. CAA is often used where the possibility of solution retention in holes, recesses and crevices exists. High chloride content causes pitting. Over time, with part processing, the chromic acid concentration is depleted by neutralization with dissolved aluminum. Therefore, free chromic acid and aluminum content must be monitored as the bath ages. Additionally, hexavalent chromium content (free chromic acid) decreases as the bath ages, while trivalent chromium and aluminum content increase. If sulfate concentration, in the electrolyte, is too high, the resulting coating can become more transparent.
A CAA coating has an appearance that varies from opaque iridescent to dark gray depending on the type of alloy. Since CAA coatings are very thin, there is not any substantial abrasion resistance. The coating has substantial corrosion resistance and is also an excellent base for paint. Solution entrapped in faying surfaces does not promote corrosion.
Increasing the temperature increases the electrolyte conductivity resulting in a substantial increase in coating weight over a given time period. However, even though the coating weight is higher, there is more porosity and decreased corrosion resistance.
Chromium is reduced from hexavalent to trivalent chromium by contact with any organic. Because of this, there will always be some trivalent chromium (Cr+3) in the anodizing bath. If there is too much Cr+3, the anodic coating will darken and may even pit. If the area of the cathode is excessive, in comparison to the anode (>5% of anodic area), reduction will also be favored.
Cr+6 à Cr+3
Also, low cathodic current density favors reduction. The cathode can be shielded to mitigate the amount of reduction occurring. Ion exchange is employed as a maintenance process that can be used to remove both excess trivalent chromium and aluminum.
Boric – Sulfuric Acid Anodizing
Like CAA, Boric - Sulfuric acid anodizing (BSAA) is used to form coatings on structural parts and BSAA coatings also have little or no effect on the fatigue strength of finished parts because they are very thin. It is well known that CAA produces a chromium mist that is hazardous to health if inhaled. BSAA is an alternative that eliminates this concern and the need for mist control. In 1990, the Boeing Company developed BSAA as a direct replacement for CAA due to pressing environmental and safety concerns. The resulting patent (US 4,894,127) has equivalent or better fatigue life on aircraft structures. Another patent (US 6,149,795), that followed in 2000, exhibits that and that the addition of an organic acid can mitigate bio-contamination.
Sulfuric Acid Anodizing
Conventional sulfuric acid anodic coatings are thick enough that they reduce the fatigue characteristics of an aluminum alloy, so they are not used on structural parts. Although the majority of the anodic coating is composed of aluminum oxide (Al2O3), there is about 10-15% of SO3 incorporated into the coating. Consequently, there is a potential for corrosion when there is solution entrapment in part faying surfaces.
On most aluminum alloys, the sulfuric acid anodic coating is colorless and transparent. Alloys containing high manganese and silicon levels tend to give grayish or brownish colored coatings. The transparency of the coating decreases with increasing coating thickness.
Small changes in operating conditions can be detrimental to the coating, especially electrolyte temperature. Lower temperatures and dilute solutions favor harder coatings. Higher temperatures and more concentrated solutions favor dissolution and may produce coatings with a soft, powdery, or spongy surface. The surface layers will be less resistant to abrasion and may rub off easily.
High chloride levels in the electrolyte will cause pitting during anodizing. High aluminum content may cause aluminum sulfate to precipitate out onto the cooling coils, heat exchangers, and piping. This condition may then interfere with agitation and temperature control
If grease or oil is in the SAA bath it mostly will exist on the fluid surface and will be transferred onto the coating surface and cause staining. If grease or oil is present on the parts prior to anodizing, a coating may not even be able to form.
Thin Film Sulfuric Acid Anodizing
Thin Film Sulfuric Acid Anodizing (TFSAA) meets or exceeds the requirement of MIL-A8625F, Type IIB. Comparatively, SAA (per MIL-A-8625, Type II) is a thick porous coating and is easy to dye color but has relatively poor corrosion resistance and an adverse effect on latent metal flexural fatigue.
TFSAA is an improvement over conventional SAA in terms of corrosion resistance and as a base for paint adhesion. These anodize properties enhancements were achieved by reducing the concentration of the sulfuric and thereby lowering the electrical conductivity. This in-turn reduced the generated heat to an ambient level and also decreased the rate of the oxide growth at the anode resulting in a finer grain and also a thin film of aluminum oxide on aluminum parts.
General Processing Overview (More exact details are in the OEM specifications)
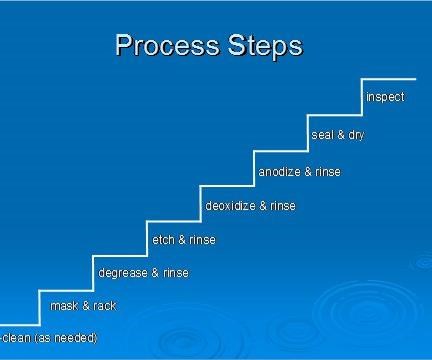
A general overview of the anodizing process is identified in the steps below. The flow diagram highlights the steps of the anodizing process and the order they are generally performed.
Metal Finishing – Buffing, polishing, wire brushing, and sanding are performed first.
Preclean – If necessary, the parts are cleaned to remove tenacious soils or markings. For example, some ink markings can only be removed by solvent wiping.
Mask - Masking is needed to cover areas that don’t require an anodic coating. For example, masking might be needed if one side of the part is anodized and the other side conversion coated. Tape and liquid maskants are commonly used products and specification listed.
Note: Using two different finishes is not recommended because of the time and labor required.
Degrease - The parts are degreased if oil or grease is present. Either aqueous or vapor degreasing can be used.
Alkaline Clean - Parts are then alkaline cleaned to remove fingerprints, perspiration, and other soils picked up during handling.
Deoxidize – Acids are used to remove oxides (of varying thickness and composition) from the surfaces of the parts. An aluminum oxide layer that is thin and of uniform thickness forms naturally on the part surfaces after withdrawal from the deoxidizing tank. An oxide layer forms when the deoxidized metal is exposed to air or water, which contains oxygen. The new natural oxide etches evenly during anodizing, promoting the formation of an anodic coating that has uniform thickness and appearance.
Alkaline Etch/Desmut– Caustic formulations are used in place of or in addition to alkaline cleaning to remove stains, thick oxides, smeared metal, shot peen residue, and other surface or embedded contamination. When aluminum is exposed to caustic chemicals (e.g. NaOH), smut is formed on the part surfaces. Smut has an appearance that varies from small black specs to totally blackened surfaces, depending on the type of alloy etched. Smut is made of alloying elements such as iron, silicon, magnesium, manganese, and copper that are not soluble in alkali solutions and are reduced during etching. Removal of the smut requires a highly acidic formulated solution, commonly known as a Desmut solution. In tanklines where a minimal amount of etching is performed, the deoxidizer is used for desmutting and deoxidizing. In other lines, where a lot of desmutting is performed, deoxidizing tanks are usually dedicated to desmutting and not used for deoxidizing. Deoxidizing is not usually required after desmutting.
Note: Alloys containing magnesium tend to build up a layer of magnesium oxide on the surface during high temperature heat treatment or during storage in humid conditions. These oxides are insoluble in alkaline solutions, and alkaline etching may result in a very rough and patchy surface as the oxide is removed by undercutting. It is advisable to deoxidize parts with magnesium oxides (nitric or sulfuric acid) prior to etching. Care should be taken to avoid over etching. Excessive etching will increase the susceptibility of some heavy metal containing alloys to stress-corrosion. Also, the aluminum surface should not be rough after etching. If the surface is rough, a thinner anodic coating will be formed (for a given set of operating conditions). This negatively affects the appearance of the coating and its corrosion resistance and is why coarse ground surfaces are generally polished before anodizing.
Anodize - Once surface preparation is complete, the parts are ready for an electrolytic conversion coating. Before a load is moved to the anodizing tank, be sure the lip boards and surrounding areas of the tank are clear. If the rack contacts surrounding structures or equipment, the system could be short-circuited.
During anodizing, the parts must be securely racked to prevent movement. The voltage is applied within seconds after the parts are placed in the anodizing solution and voltage is slowly ramped up to the steady state value. If the voltage isn’t applied immediately, a smut may form on the parts and interfere with formation of the anodic coating once the voltage is applied. The voltage is held constant for the required time in the last stage of the ramp-up.
When anodizing is complete, the voltage is stopped, and the parts are need to be removed from the anodizing solution within an allowed tolerance but there should be no dwell after the current stops. The sooner the load can be removed from the anodize tank, lessens the amount of etching caused by the acid solution on the newly formed oxide. Also, it is important to note that the anodic coating does absorb some solution, trapping acid. Thorough rinsing is performed, after withdrawal from the anodize tank, to remove the acid solution. If acid solution is allowed to dry on the parts, it can interfere with subsequent paint adhesion.
If wet parts need to be handled during the process, clean latex rubber gloves should be worn. Clean fabric gloves should be used for handling dry parts.
Rinsing- Rinsing is performed after most of the processes: aqueous degreasing, alkaline cleaning, alkaline etching, deoxidizing/desmutting, and anodizing. Prior to rinsing, the parts are held above the respective processing solutions to allow drainage back into the solution. The parts are then transferred to the rinse tank before they begin to dry. Chemicals that dry on the parts may become insoluble and interfere with subsequent processing. If rinsing is not thorough, chemicals remaining on the parts will be carried on to the next solution and could reduce the effectiveness of that solution.
Seal/Dry/Paint – After anodizing, the parts may be dyed, sealed, or simply dried.
Dyed - Anodic coatings are dyed directly after anodizing. After dyeing, a special seal is used to set or seal the dye in the anodic coating, and then the coating is dried.
Sealed - If paint will not be applied in a timely manner, the anodic coating is sealed to prevent it from becoming dirty. It is possible to under or over seal the anodic coating. Over sealing will cause paint adhesion failures. If not painted, under sealed coatings will have compromised corrosion protection.
Unsealed - Parts that are going to be painted in a timely manner are not sealed because sealing reduces paint adhesion. These parts are transferred directly to the dryer after rinsing.
Dyeing
To dye an anodic coating, the parts are immersed in a solution of de-ionized water and organic dye. The anodic coating adsorbs the dye: aluminum ions in the coating act like chelating agents and grab and hold onto the dye molecules. Generally, parts are immersed in the dye until the pores of the anodic coating are saturated. To achieve this level of saturation, the temperature, pH, and concentration of the dye solution and the immersion time must be strictly controlled.
Thicker anodic coatings, such as those produced by conventional sulfuric acid anodizing, adsorb the dye better. The amount of color achieved is dependent on the depth of the anodic coating pores. The pores have to be deep enough to adsorb enough dye to achieve the color. For uniform color, the anodic coating must be of uniform thickness and pore structure. After dyeing, the anodic coating is usually sealed in a nickel acetate solution to ensure weather resistance and color-fastness.
To make up the dye solution, dye is dissolved in de-ionized water at the recommended concentration and pH. The dye solutions are used at room temperature or heated up to around 150 °F. Immersion times vary from 5 to 30 minutes; longer times produce deeper color.
In an attempt to summarize the basic requirements involved for the main Aerospace Anodize processes the following table has been constructed which provides categories common to each process. These categories are: anodize, seal, anti-mist, polyballs, make-up water, rinse, contamination, power capability, racks, cathodes, agitation, dry, appearance, quality control, and paint.
Table 1: Anodize Process Comparison
|
CAA
|
BSAA
|
PAA
|
SAA
|
TFSAA
|
|
|
Anodize
|
* Make-up: ~35lb of CrO3/100 gal of soln.
* Temp: 95 + 5°F
* Chemistry: Free CrO3 and total Cr+6
* Voltage: 22 + 2V
* Initial: 5V
* Ramp rate: 5-7V/min
* Time: 22-60 minutes
|
* Make-up: ~2.5lb H2SO4/100 gal; ~6.7lb H3BO3/100 gal
* Temp: 80 + 4°F
* Chemistry: H2SO4, H3BO3, Al, Cl, & Cr
* Voltage: 15 + 1V
* Initial: 5V
* Ramp rate: 5V/min maximum
* Time: 18-22 min
|
* Make-up: 13-16 oz/gal
* Temp: 77+ 5°F
* Chemistry: H+, Cl, F
* Voltage: 15 + 1V
* Initial: 5V
* Ramp rate: 2-7.5 V/min
* Time: 20-25 minutes
|
* Make-up: ~11lb H2SO4/100 gallons of water
* Temp: 68-72°F
* Chemistry: H2SO4, Cl, F, Al, Fe & Cu
* Voltage: 16 + 6V
|
* Make-up: 3-5% H2SO4 (by weight)
* Temp: 80 ± 5°F
Voltage ramps 3-4 volts per minute and hold for 15 volts for 20 to 25 minutes until the proper coating weight is achieved on 2024-T3 aluminum
|
|
Seal
|
* Make-up: ~26g of CrO3/100 gal of soln
* Temperature:
Dilute Cr,195 + 5°F
DI, 150°F minimum
* Chemistry: Cr+6, pH, & silicates
* Time: 23-28 min
|
* Make-up: ~26g of CrO3/100 gal of soln
* Temperature: 195 + 5°F
* Chemistry: Cr+6, pH, & silicates
|
none
|
Dichromate
* Make-up: 50lb Na2Cr2O7×2H2O/100 gal
* Temperature: 9180°F
* Controlled: Na2Cr2O7, pH, & Cl
* Time: 14-16 min
Note: Parts require rinse after seal
Dilute Chrome
* Make-up: ~26g of CrO3/100 gal of soln
* Temperature:
Dilute Cr,195 + 5°F
* Chemistry: Cr+6, pH, & silicates, & TDS
* Time: 23-28 min
DI water
* Temperature: 160 -210°F.
|
* Make-up: 75-125 ppm CrO3 or Na2Cr2O7
* Temperature: 185-195°F Note: Allow to dry without rinsing
|
|
Anti-Mist agents
|
* used to control air emissions of Cr
* adjust agitation so that excessive foam isn’t generated
* increase in anodize time might be needed
|
Not used
|
Not used
|
Typically not used due to sufficient ventilation systems, may be applied if current ventilation system is not sufficient.
|
Not used
|
|
Polyballs
|
* Use only solid molded, linear HDPE
* LDPE and PP cannot be used
|
HDPE recommended
|
Not needed
|
HDPE recommended
|
Not used
|
|
Make-up water
|
Controlled: total solids, Cl, F, & pH
|
Controlled: total solids, Cl, F, & pH
|
Controlled: total solids, Cl, F, & pH
|
In most cases plant water will be sufficient for make-up water.
|
Reverse osmosis or DI
|
|
Rinse
|
* Temperature: <95°F
* TDS: 1st rinse, 5000ppm; 2nd rinse, 1000ppm
* pH: 2.5 – 8
* Time: 0.5-15 min
|
* Temperature: <95°F * * * TDS: 1st rinse, 5000ppm; 2nd rinse, 1000ppm
* pH: 2.5 – 8
* Time: 3-15 min
|
* Temperature: 110°F max
* TDS: 1st rinse, 5000ppm; 2nd rinse, 1000ppm
* pH: 2.5 – 8
* Time: 5-15 min
|
* Temperature: <90°F
* TDS: limit is set at 150 ppm over that of the water makeup; 350 ppm TDS is allowed in a rinse shared between the anodize & deox
|
No rinse impregnation of the seal
|
|
Contamination
|
* control amount of Cl and sulfate
* BaCO3 powder is used to remove sulfate contamination
|
* prone to bio-contamination
* use of sodium benzoate allowed
|
* Controlled: Cl, F
* filtering required to remove fungus
|
Controlled: Cl, F
|
100 micron string wound polypro filters may be required to control fungi
|
|
Power Capability
|
control the voltage to within +2V
|
* auto-ramp rate of 3-5V/min
* control the voltage to within +1V
* minimize ripple
|
* ripple, 5% max
|
16-22V , up to 18% ripple
|
* ramp rate of ~3V/min
* 15V for 20-25 minutes
|
|
Racks
|
Al, Ti, Al with Ti-tips
|
Al, Ti, Al with Ti-tips
|
* PTSV of 6V min
* 8amps/sq ft min
|
Al, Ti or a combination
|
Titanium
|
|
Cathodes
|
* integral: walls of a steel anodizing tank
* external: Pb or CRES
* auxiliary cathodes: used if the ratio of the L/D of an interior surface or hollow member is greater than 8
|
* integral: walls of a 316L or 304L SS tank * external: Pb, Ti, or CRES
* auxiliary cathodes: used if the ratio of the L/D of an interior surface or hollow member is greater than 8
|
* stand-off insulation recommended
|
Pb, Ti, or CRES cathodes must have a surface area greater than ½ the surface area of the work load.
|
316 SS or Ti
|
|
Agitation
|
Required when power is on
|
Required when power is on
|
Allowed during anodizing
|
Required when power is on
|
Mild air sparging
|
|
Dry
|
Temperature:
* Sealed, 190°F max
* Unsealed, 160°F max
|
Temperature:
* Sealed, 190°F max
* Unsealed, 160°F max
|
Temperature:
* 160°F max
|
* Temp: 140°F max
* Time: 30 min max
|
No rinse and air dry with latent heat
|
|
Appearance
|
*color is light gray prior to sealing
* golden tint after sealing
* Defects: powdery areas, arc burns, breaks, scratches, pits
* solution bleed-out is evidence of base metal cracks
|
*color is light gray prior to sealing
* golden tint after sealing
* Defects: powdery areas, arc burns, breaks, scratches, pits
* solution bleed-out is evidence of base metal cracks
|
* Defects: smut, stains, burned areas, scribe lines, scratches, pits
* inspect for anodize at grazing angle and polarizing filter
|
* continuous, smooth, adherent, and uniform
* Defects: powdery areas, arc burns, breaks, scratches, pits
|
Light yellow
|
|
Quality Control
|
* appearance
* solution chemistry
* water purity
* air cleanliness
* voltages
* temperature
|
* appearance
* solution chemistry
* water purity
* air cleanliness
* voltages
* temperature
* coating weight
* seal time posted
* salt spray
* anodize time
|
* Qualification required per D-16925
* appearance
* solution chemistry
* water purity
* air cleanliness
* ripple
* voltage
* anodize time
* wedge crack
|
* appearance
* solution chemistry
* water purity
* air cleanliness
* voltages
* temperature
* coating weight
* seal time posted
* salt spray
* anodize time
|
* salt spray
* coating weight
|
|
Paint
|
Within 16 hours
|
Within 16 hours
|
Move to controlled contamination area within 3 hours after final rinse
|
Within 8 hrs after final rinse.
|
Within 8 hrs after final rinse.
|
Equipment
Since anodizing is an electrolytic process, it requires more auxiliary equipment than most tankline processes. The figure below shows the basic components of an anodizing cell. To anodize, you will need the following equipment and sub systems listed below:
- Process Tanks
- Rectifier (supplies DC for anodizing)
- Current Pathway (connects the positive side of the DC power supply to the anode, hood/parts/racking; connects the negative side of the power supply to the tank or other metal structure, which serves as a cathode.)
- Anode (hood/racking/parts)
- Cathode (tank walls or plates that hang in tank)
- Agitation (to dissipate heat generated by process)
- Heating (to maintain temperature within range)
- Cooling (to cool electrolyte which heats up during anodizing)
- Rinse Tanks
- Seal Tank
Process Tank
The anodizing tank is sized based on the size and configuration of the parts, the number of parts processed per load, the size and configuration of the hood and racking, and the amperes of rectification needed for anodizing. As a general rule of thumb, one gallon of electrolyte is needed for each ampere of rectification. The tank also has to be designed to accommodate the auxiliary equipment needed for anodizing, such as heating, cooling, agitation, filtering, a means to support the hood, and anodic fixtures. The tank should be capable of supporting the hood and/or racking during anodizing. The crane is usually disconnected from the hood or load to prevent current flow to the crane and to free up the crane for other uses because the anodizing process is quite long.
Anodizing tanks- can be constructed out of steel, mild steel or stainless, or non conductive materials depending on the process you need to run. Most CAA Tanks are constructed of mild steel using the tank walls and bottom as the cathode with shielding. BSAA tanks are typically constructed of 316 SS with the tank walls and bottom used as a cathode, and can also be provided as a polypropylene (PP) tank with a Stainless Steel (SS) cathode system. SAA tanks are typically constructed of 316 SS reinforced with a PP drop in liner or an acid-resistant material such as polyvinylchloride (PVC) or rubbers such as neoprene. Auxiliary cathode systems can be constructed of lead, Ti or CRES, but the most commonly used cathode material used today is aluminum. Fiberglass structure should not be used inside the tank because it serves as a host to Alternaria fungus, which is sometimes problematic in BSAA tanks.
Rectifier - Electric power that is purchased from utility companies is usually in the form of alternating current (AC); the voltage is too high and the amperage is too low to be used for anodizing. A rectifier transforms alternating current into direct, unidirectional current similar to that provided by a battery. Rectifiers allow current to flow freely in one direction and offer high resistance to flow in the opposite direction. Rectifiers also have step-down transformers that reduce the voltage and increase the amperage. If a rectifier is wired properly, it will deliver direct current that is almost as constant as that from a battery. The rectifier control panel generally has readouts of the operational voltage and the amperage being drawn. If the rectifier is being operated manually, adjustments will have to be made throughout the anodizing process to keep the voltage constant. Most rectifiers can be programmed to automatically ramp and hold the voltage for the anodizing time. In voltage control setting the current limiter should be high to allow the current to optimize based on surface area and conductivity of the solution. The rectifier control panel may be placed near the tank, and the rectifier may be located remote from the tank in a safer location, if necessary. The rectifier is sized based on the surface area of a part load and the current density required.
Anode (Racking/Parts) – Generally, to conduct current from the rectifier to the parts, permanent fixtures installed on the hood are brought into contact with other fixtures that are permanently installed on the anodizing tank. Current is conducted via these fixtures to an aluminum or a copper bar carried by the hood. Racks are securely attached to this bar, which distributes current evenly among the individual racks. The racks are clamped, wired or bolted to the bar.
Racking for anodize is slightly more tedious than racking for other processes. The parts must be individually attached to the rack with the use of special fixtures. These fixtures vary among the different tanklines and include clamps, clips, wire, and bolts. These fixtures in turn are bolted, screwed or attached by another means to the rack. The main function of these fixtures is to form a positive contact with the parts to ensure good electrical contact through out the process. Normal handling of the racks should not cause damage to the parts or loosen the contacts. If the part moves at the racking point during anodizing, the anodic coating will not form properly and damage to the part (arc strikes) may occur.
For all immersion processes, parts need to be racked to promote drainage and prevent entrapment of air. Wherever air is entrapped, the anodic coating will not form.
Several or numerous contact points may be needed to secure a part from movement. In general, each contact point should not be allowed to carry more than 10-20 amperes for smaller parts and 60 amperes for larger parts, depending on the gauge and surface area of the part. If the fixtures that secure the parts/racks exert uneven forces, the anodic coating thickness will not be uniform throughout the load. If parts are racked in a stressed position or with different alloys on the same rack, the anodic coating might be nonuniform on a given part.
The positioning of the parts within the tank also has an effect. A rack in the center of a tank may not draw as much current as a rack located at the sides, near the cathodes. Also, for a given rack, parts on the bottom of the rack might draw more current than those on the top. These racking differences could result in non-uniform coating thickness between racks of parts and between parts on a given rack. The tank and racking system are usually designed so that this variation is within the tolerance of the process. Since an electrical resistance film is being created thinner coated areas will self adjust to achieve overall uniformity (Ref. 2)
Racks should be designed to allow reasonable spacing between parts and to hold them parallel to the cathodes. Although the throwing power of most anodizing solutions is good, variations in anodic film thickness will occur if the work is too densely packed or is at very different distances from the cathodes.
Parts need to be racked securely to ensure electrical contact. Both the racks and parts form insulating coatings during anodizing; contact between the racks and parts must be secure with some pressure. Thicker films tend to separate the rack from the work, especially is there is a local rise in temperature in that area. If the part becomes insulated from the rack, extra load is transferred to other contacts and arcing or burning can occur. If the parts move on the racks during anodizing, arcing can occur at one or several of the contact points, also resulting in burn marks at or near the contact points. When racking a part, use a minimal number of contacts. Electrical contacts should be made in non-critical regions of the parts whenever possible, typically in part excess areas.
The racking material should not have lower electrical resistance to current flow during anodizing than the parts to avoid receiving higher current density and stealing current from the parts. For this reason, alloy 2024-T6 is sometimes used for racking. When other aluminum alloys are being anodized, lower current density is produced on racks fabricated from alloy 2024, and minimal current is robbed from the parts. Also, the thin anodic films produced on this alloy require minimal stripping prior to re-use, which results in longer rack life. Another combination that may be used is 2024-T6 with titanium contact tips or titanium-clad aluminum tips. If made from aluminum, the rack material should be on the same or a similar alloy type to the material being anodized, or of a material that does not tend to rob the work of current by consuming more than its share. Aluminum alloys 6061-T6, 6063 and 6082 work well and provide good spring contact with positive pressure and don’t contain copper, which builds up in the tank.
Although titanium is a poor conductor and is expensive, it has the advantage of remaining conductive and not dissolving during the anodizing process. A thin passive oxide film is formed on titanium during anodizing; this film prevents the titanium from drawing significant current, yet permits the current to flow to the aluminum part. The film is soft and is usually broken through when racking, so it doesn’t require frequent stripping. However, the film should be stripped periodically with hydrofluoric acid to ensure the contact points provide good electrical contact.
Aluminum racks- must be stripped after each cycle to remove the anodic coating formed during anodizing. (The anodic coating is an insulator and will reduce the conductivity.) Caustic soda solution (2-15 oz/gal) at 130-160 °F is commonly used for stripping the racks. The racks are then desmutted. With repeated usage, the racks become thinner and weaker. Eventually, they must be disposed of. Expensive racks can be stripped in a chromic-phosphoric acid solution, which only dissolves the anodic oxide coating and not the base metal. Molten salt baths, which operate at around 750 °F, are sometimes used for stripping racks. Abrasive blasting can also used to strip the anodic coating from racks.
Basket Anodizing - For smaller parts that will not nest, basket anodizing is sometimes used. (Note: anodic coating will not form at the contact points between nested parts.) Here, parts are placed in plastic or metal perforated baskets similar to that shown in the figure to the right. The basket is clamped to the bus bar just like the other racks. Many parts can be anodized at once, but the coating thickness on the parts may vary throughout the basket.
The baskets are made of perforated aluminum, titanium, or plastic. The perforations allow degassing, drainage, and heat dissipation. In some designs, the solution is pumped into the basket to help maintain uniform temperature.
An aluminum rod runs down the center of the basket and carries current to the parts. The rod needs to be large enough to carry the anticipated current to the parts. Electrical contact is made between the parts and the aluminum rod and through part-to-part contact for parts not in contact with the rod. The basket has a lid that can be adjusted to fit snugly against the parts. The parts must be held rigid to withstand vigorous agitation and to prevent arcing due to make and break contact. Part movement will result in the buildup of the oxide coating at previous contact points and the resistance will be too great for current flow.
The areas where the parts contact one another and the rack and rod need to be small since no anodizing occurs at these areas. Parts must be racked to avoid air pockets; anodic coatings will not form unless the solution is in contact with the part surface.
Cathode – The walls of a metal tank usually serve as the cathode. If the tank is constructed of or lined with a nonconductive material such as plastic, cathodes will need to be provided. The cathodes are usually designed to have a surface area greater than one half of the surface area of the largest load that will be anodized. The cathodes can be constructed with 304, 316 or 321 stainless steel or commercially pure titanium. The anodes (parts and racks) cannot contact the cathodes during anodizing or the system will be short-circuited.
The tank walls have to be smooth with minimal protruding structure. If used to support auxiliary equipment inside the tank, unistrut will corrode preferentially because it protrudes from the tank walls. It is typical for equipment inside the tank to be shielded from the parts by plastic grating.
Internal cathodes are needed to anodize parts that have deep holes or cavities with length to diameter ratios greater than 8:1. (This ratio may vary depending on the type of anodizing.) Internal cathodes complicate the racking and increase operational costs. Internal cathodes are attached to the bar along with the racks.
Heating, Cooling, and Agitation – Anodizing specifications require the temperature of the electrolyte or anodizing solution to be maintained within a limited range. If the temperature drifts out of range, it could be detrimental to the anodic coating. Heating and cooling systems maintain the solution at constant temperature, while agitation ensures uniformity of temperature and concentration throughout the tank.
There are number of reasons why temperature control of the anodizing solution is needed, which include:
Foremost, the temperature tolerances are usually tight. Without controls, the temperature of the solution may vary throughout the year. In the cooler months, the solution temperature might be ~65 °F, while in warmer months; it might even approach ~85 °F.
The oxidation reactions that occur during the formation of the anodic coating are exothermic, which means the reactions produce heat. If this heat isn’t dissipated, the temperature at the part surface will increase. High temperatures allow the electrolyte to attack the coating more aggressively, and the result will be a soft or spongy anodic coating.
Variations in conductivity due to temperature a temperature gradient will change the appearance of the anodic coating. To ensure uniform appearance of the coating among a load and between part loads, the temperature needs to be uniform throughout the tank and as constant as possible during anodizing.
A means of heat exchange, such as steam pipes, plate coils, or hot water pipes is used to heat the anodizing solution. The heat exchanger may be placed inside or outside of the tank. If place outside of the tank, the solution is pumped to the heat exchanger and then returned to the tank.
[Note: For BSAA and TFSAA, if the heat exchanger is placed in the tank, the incoming steam pipe to the heat exchanger should be fabricated with Carpenter 21 (high nickel alloy steel). Other materials have failed due to corrosion of the pipe where it enters the anodizing solution (air/liquid interface). Commercially pure titanium or 316L stainless steel heating coils can be used.]
Cooling may be supplied by coiling coils that are installed inside the tank. Plant water or chilled water is supplied to the coils. In some applications, the anodizing solution is pumped through a refrigeration unit.
Air agitation sparging- is commonly used to agitate anodizing solutions. Compressed air is forced through perforated PVC or polypropylene pipes mounted on the bottom or sides of the anodizing tank. The compressed air needs to be free of water, oil, and solid particles. Blowers are usually of the positive displacement or centrifugal type, which produce large volumes of low-pressure air. Compressors are more likely to give variable agitation along the length of the bath. The literature (Ref. 3)recommends 0.7 to 1.5 cubic feet of air per square foot of tank surface.
Pump recirculation may also be used to agitate the solution. The intake and outlet of the pump are located within the tank, and the anodizing solution is pumped through a manifold mounted on the bottom or sides of the tank. Mechanical agitation of the bus bar is common in plating operations, but is rarely used in anodizing because the movement has a tendency to loosen the contacts.
Ventilation
Testing-
Aerospace anodizing involves several test requirements as quality control checks based on the multiple functionality provided by the anodic coating.
The thickness and uniformity of an anodic coating are important because they are directly related to corrosion resistance. Generally, thicker anodic coatings have better corrosion resistance. Anodic coating weight is a direct measure of the thickness. Anodic coating weights vary among different anodizing processes. The following coating weights are exhibited for comparison only.
|
Type of Anodize
|
Coating Weight (mg/ft2)
|
|
Chromic acid
|
200
|
|
Boric sulfuric acid
|
200-700
|
|
Conventional sulfuric acid
|
1000
|
|
Thin film sulfuric acid
|
300 to 700
|
|
Phosphoric acid
|
100
|
Coating Weight determination is performed per ASTM B137 and is performed at a scheduled frequency.
Salt Spray testing is typically required for “sealed” anodize. This test is a measure of corrosion resistance and is performed per ASTM B117. This test requires a salt fog cabinet, a DI source, and trained QA operators. Most noteworthy is that this evaluation typically requires 336 hours, so a salt spray failure is particularly problematic.
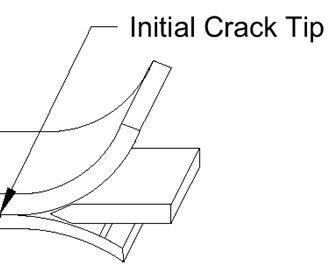
Wedge Crack testing is a test specific to PAA and is performed to evaluate bond integrity. The evaluation involves exposing pre-cracked bonded specimens to high humidity and warm to hot temperatures for a set time.
This evaluation also requires primer application capability, a clean room lay-up area and an autoclave for cure of the prepared specimens bonded with an epoxy film adhesive.
Part to Solution Voltage (PTSV) is another PAA specific evaluation and is performed by attaching one end of a solid copper wire to a voltmeter at the negative terminal and placing the other end of the wire approximately 1 inch away from the aluminum surface area that is to be measured (Figure 3 of BAC5555). This test is to be performed as a check that the rectifier is adequate for delivering power for any new major racking configuration changes.
Paint Adhesion evaluations are performed in accordance with the process specification specific to the primer being applied and most often is essentially a tape pull type of test. Typically, the paint adhesion test involves a controlled cure or ambient conditioning the part or paint adhesion panels over a specified time period (oftentimes seven days). The tape pull test is performed per a testing standard (BSS7225) and can involve a patterned scribe of a test panel. If testing is performed directly on the part, there is no scribing prior to the tape pull. Additionally, the part or panels can be tested “dry” or “wet”. The “dry” testing can be performed as soon as the specimen is cool after cure. The “wet” testing required exposure to water for a seven day period prior to the tape pull. The frequency required for tape pull paint adhesion testing is again established in the primer specific process specifications which most often specify “QA discretion” but can also require a regimented sampling plan that that is less frequent based on the number of successful consecutive evaluations.
An alternate form of paint adhesion testing is known as aluminum rod adhesion test or “craze rod” and simply involves dragging an aluminum rod over an aluminum surface at a specified angle and approximate pressure. Subsequently, the test area has a specific tape applied and is then pulled for evaluation of the amount of paint removal.
Known Pitfalls
Fungal Growth in BSAA Solution
A fungus called Alternaria commonly contaminates BSAA solutions. When the fungus levels exceed 10 colonies per ml on a microbiology colony count test, the BSAA solution becomes visibly cloudy. The actual number of colonies may be larger because clumps of the fungus are present.
Alternaria fungus deposits on the parts during anodizing as small black particles or clumps. This can prevent small areas on the parts from anodizing and sometimes interferes with downstream processing. The small particles are removed in the rinse tank, but large clumps can remain on the parts and, if painted over, will interfere with localized paint adhesion. The larger clumps can be removed by spraying with a water hose after anodizing, which is impractical for some part configurations or if tank access is poor.
When BSAA solution with fungal contamination is viewed in a glass container, the fungus has the appearance of small black particles. Alternaria colonies stick together and form particles, balls and long streamers, up to several inches in size. If contamination is extreme, the Alternaria may agglomerate and form mats on solution surface. The fungus is distributed throughout the solution and clings to the tank walls and fiberglass grating. It is difficult to remove from the tank walls and grating. If not removed, it will rehydrate upon recharging and continue to be a nuisance.
Although the Alternaria fungus is ubiquitous the most significant source of fungus in a newly charged tank is the un-removed build up of fungus and active spores on the tank walls leftover from the previous solution. If practical, the old solution should be spiked with a fungicide before new solution is charged. A fungicide actually kills the fungus.
Alternaria reproduces by releasing spores, which are airborne. The spores are several microns in diameter by about 20 microns long. Filtration (10 micron) can be used to remove mature fungi, but the smaller spores are too small to filter economically. A fungistat can be added to the solution to control fungal growth. The fungistat doesn’t kill the fungi or spores, but its presence prevents the spores from growing and reproducing. If the fungistat is depleted, the spores will begin growing again and reproducing. For this reason, an effective concentration of fungistat must be maintained in a problematic BSAA solution at all times. The fungistat should be added within several days after charging a new BSAA tank and as the solution ages to maintain the concentration. The foam generated by air sparging may look dirty in a BSAA solution contaminated with fungus. After fungistat is added, the foam returns to its normal white color.
Benzoic acid and sodium benzoate fungistats have been tested for use in BSAA solutions. Benzoic acid has limited solubility in cooler water (~80 °F), so it needs to be mixed with warm water to increase its solubility prior to making an addition. Sodium benzoate is preferred since it has better solubility properties as compared to the conjugate acid. Refer to the specification for details on using this fungistat. The concentration of these fungistats can be measured using UV-Vis spectrometry. The double carbon bonds in benzoic acid measure at 242 nm wavelength.
A more thorough examination of metal finishing bio-contamination conditions is available per the paper entitled, “Bio-contamination Control in Metal Surface Finishing Operations” (Ref. 4).
Bacterial Growth in BSAA Rinse Tanks
Bacteria have also been identified in BSAA rinse tanks. Bacteria introduction into the water source is likely due to poorly maintained pre-treatment carbon filter beds. The bacteria was first noticed as small brown spots appearing on anodized surfaces after rinsing in the bacteria contaminated rinse water. These brown spots or stains could be removed by cleaning with water in accordance with BAC5632, but was too time consuming to be practical in production. The rinse tank had to be dumped and cleaned. The remedy for such a condition can be to spike a rinse tank to be dumped with peroxyacetic acid (trade name Purisan) at ~50ppm and left to recalculate for ~8 hours before dumping. Furthermore, sodium benzoate can be used as an in-situ fungistat for the first rinse of a 2-rinse configuration.
Chromic Acid Anodizing Stains
Stains began appearing on parts when sealing before painting was eliminated. The cause of this staining was bleed out of chromic acid anodizing solution from faying surfaces in the racking. The staining did not occur when the parts were sealed because the seal (25-minute high temperature soak) rinsed out the solution trapped in the faying surfaces. It was determined, through paint adhesion testing and chemical analysis of the stains, that the stains were not detrimental to paint adhesion. A Process Specification Departure (PSD) was issued to BAC5019 to allow light residual chromic acid stains from straps or part hangars.
Salt Spray Failure
Sealed anodize processes can be subject to a corrosion related test known as salt spray or salt fog and is formalized as a test procedure per ASTM B 117. Failing results are not uncommon and can be rather difficult to solve due to the many potential root causes. Due to the 336 hours test period (two weeks) making a first time correct diagnosis and corrective action plan imperative. Presented at several national conferences and first published in 2005, the paper, “Salt Spray Failures on Anodized Aluminum” (Ref. 5) categorizes root causes in the form of a proven troubleshooting guide. It is essentially a tabular checklist, categorized mostly by process solution, but also includes sections on other non-tankline processes, equipment, and salt spray test conditions. Although this paper focuses on boric acid sulfuric acid anodizing, it can be applied to other anodizing processes that can guide process engineers to quick and effective remedies.
Paint Adhesion Failure
Even when applied in a factory setting, the inability of a paint primer or topcoat to adequately adhere to an airplane substrate is a topic that periodically has required the priority and expertise of much engineering and analytical resources. The many causes of factory aerospace paint adhesion problems can make identifying root causes very difficult. Typically, paint adhesion problems are the result of contamination between the paint and the substrate surface. Specific to the anodize process, paint adhesion failures can be caused by any one of the surface process steps (see Figure 2).
The rule of thumb is that the more critical chemical processes are in reverse order of operation: seal; anodize/alodize; acid deoxidize/desmut; alkaline etch; degrease clean. Each chemical operation has a set of concentration and pH parameters that are monitored and maintained. Additionally, the associated rinses are maintained at an optimum cleanliness (measured by total dissolved solids). These chemistries result in monitored values such as: seal hydration; anodize coating weight; and etch rate. Contamination is also a source of concern in tankline processing. However, the source of contamination is often caused by the part themselves that are being processed. For instance, alloying elements, left unchecked, can “replate” onto the part and go un-noticed until the part is paint adhesion tested. Consequently, what can therefore be very useful to troubleshooting a paint adhesion problem would to have at least a rudimentary understanding of the mechanistic chemical reactions that are occurring at each process step. As an example, the alkaline etching step can be partially described how the alloying of Copper reacts with caustic resulting in copper oxides that are insoluble.
4Cu + 2NaOH + Na2S + 3H2O à Cu2O + 2Na2CuO2 + H2S + 3H2
The acid desmut step can be partially described by how these copper oxides are made soluble by being converted to copper nitrates:
3Cu2O + 14HNO3 à 6Cu(NO3)2 + 2NO + 7H2O
CuO + 2HNO3 à Cu(NO3)2 + H2O
The important piece of chemistry to note then is that if the desmut operation is not doing its job, insoluble copper oxides can be left behind as a very thin smut layer that is not detectable by water-break-free inspection. Should this condition occur, massive paint adhesion can result and not be detected unless paint adhesion testing is performed.
Summary
In summary the authors variety of experiences provides credence that there is real business possibilities in aerospace manufacturing off-load of anodize and companion paint, adhesive bonding processing.
We see this as a real opportunity for companies that have the financial acumen and perseverance to accept this challenge and turn it into a profit.
Our intent was to present a comparison of the different current aerospace processes in use today for you to review and assist in your decide if this is a possible addition for your facility. We are hopeful that the information provided will be a valued reference to your library.
REFERENCES & ACKNOWLEGEMENTS
References
1) Marceau, Firainhoc, Moji, Method for Providing Environmentally stable Aluminum Surfaces for Adhesive Bonding and Product Produced, US Patent 4,127,451 (11/28/1978)
2) E. W. Turns, R. E. Forrester, A. C. Porter. Technical paper for “The 20 Volt Chromic Acid Anodize Process for All Aluminum Alloys. Presented to the American Electroplater’s Society, Atlanta, Georgia Technical Conference and published in the Journal of the American Electroplaters Society (Now Sur-Fin)
3) T. Turner, Proc. Aluminum Association Finishing Seminar, Paper AN-2, Aluminum Association, Washington, D.C.
4) Fullen W. J., Bio-contamination Control in Metal Surface Finishing Operations, Plating & Surface Finishing May 2003
5) Fullen W. J., Salt Spray Failures on Anodized Aluminum, Metal Finishing
6) Unangst, J Fullen, W. J., Rinse Water Rinse Calculator, Plating & Surface Finishing Dec 2004
Acknowledgment
Julie Unangst, a Boeing Chemical Engineer, was a major contributor to the content of this presentation and associated paper.
Related Content
Development of a Novel Hexavalent-Chromium-Free Aluminum Sacrificial Paint
Hexavalent chromium is a known carcinogen, repro-toxin, and mutagen. Its elimination is of high importance to the aerospace industry, which has struggled to find high performing alternatives. Legacy aluminum sacrificial paints have traditionally utilized hexavalent chromium to prevent corrosion and coatings which are equal to or better than have been difficult. This first paper discusses the novel process from the supplier point-of-view.
Read MoreVST Appoints Marketing and Communications Director
Julie Sims has been named as Valence Surface Technologies' marketing and communications director.
Read MoreHexavalent-Chromium-Free Aluminum Sacrificial Paint Validation
Hexavalent chromium is a known carcinogen, repro-toxin and mutagen. Its elimination is of high importance to the aerospace industry, which has struggled to find high performing alternatives. Legacy aluminum sacrificial paints have traditionally utilized hexavalent chromium to prevent corrosion and coatings which are equal to or better than have been difficult. This second of two papers discusses the hexavalent-chromium-free process from the user point-of-view in terms of the process validation work by Rolls Royce Corporation.
Read MoreAnodizing for Bonding Applications in Aerospace
Anodizing for pre-prep bonding bridges the gap between metallic and composite worlds, as it provides a superior surface in many applications on aluminum components for bonding to these composites.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreMasking Solutions for Medical Applications
According to Custom Fabricating and Supplies, a cleanroom is ideal for converting, die cutting, laminating, slitting, packaging and assembly of medical-grade products.
Read More