Recovery/Recycling Methods for Metal Finishers
A guide to lowering pollution and recovering valuable process constituents.
While the metal finishing sector has seen improvement over the last few years, there is still tremendous pressure to reduce costs due to future uncertainties.
In order to reduce these costs, many facilities instituted wastewater treatment improvements, including changes in wastewater pretreatment chemistry and the use of sludge dryers. While these means are quite effective in reducing the cost of treatment chemistry and off-site disposal, by themselves they are not enough.
Many localities and states are mandating lower wastewater limitations than are presently required under EPA’s Electroplating and Metal Finishing Pretreatment Standards (40 CFR 413 and 433). In addition, biomonitoring of direct discharges into surface waters has become a very common condition of NPDES permits.
Not only is biomonitoring very expensive, but many times the discharger must reduce contaminant levels significantly below the permit’s discharge limits to pass the tests.
Because of the uncertainty of future costs and squeezed profits, as well as the public and political pressure on industry to reduce toxic chemical emissions, those finishers who respond to this challenge by seriously evaluating and implementing recycling and recovery opportunities will have a great competitive edge.
First Things First
Metal finishers will agree that the most important process material, both in terms of quantity and quality, is water. Many a recovery/recycling technology has failed because of the lack of attention paid to the incoming water supply. Since many recovery technologies recover the bath process dragout from the rinses, the plating “contaminants” found in tap waters can build up to levels that could cause a failure of bath chemistry, resulting in expensive solution disposal and makeup.
Before considering the implementation of recovery methods, finishers must investigate the quality of their incoming water. Water to be used for bath and recovery rinse makeup may have to be treated to high quality.
The two most common technologies used for treatment of water are ion exchange and reverse osmosis. If one is a high water user and the incoming water has total dissolved solids greater than 500 ppm, it may be more economically advantageous to use reverse osmosis alone or as a “roughing” filter in front of ion exchange.
Another key action when preparing for recovery and recycling is to determine the least possible rinse water flow rate that can be achieved without adversely affecting product quality.
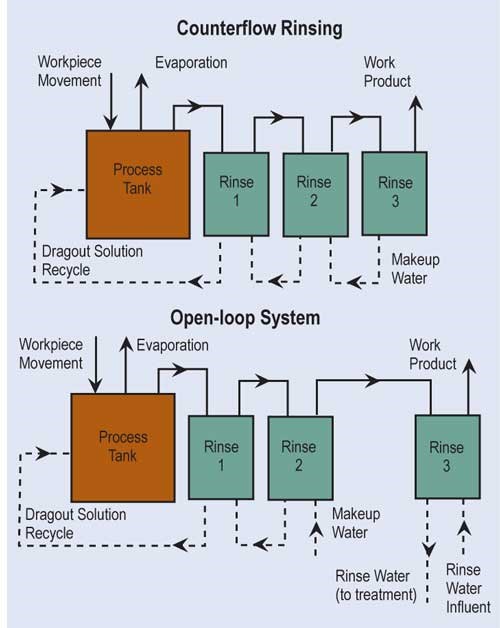
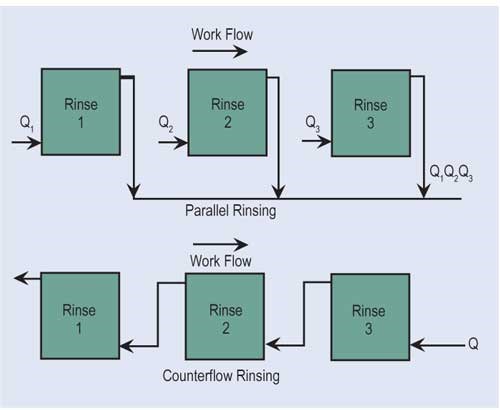
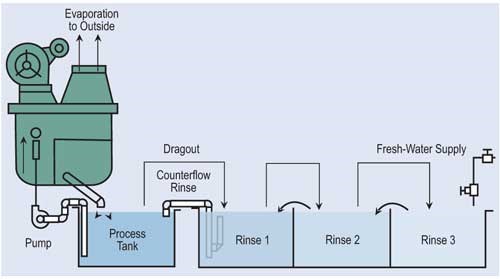
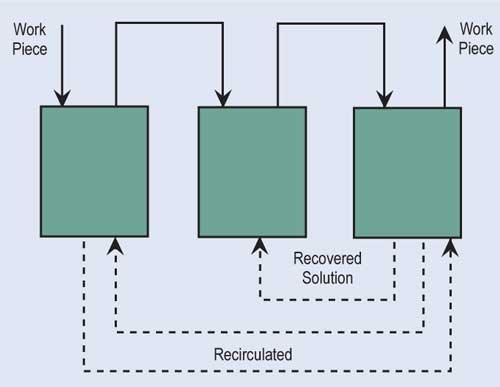
The most effective way of reducing wastewater flow is to convert parallel rinses to counterflow rinses, and, if at all possible, add additional counterflow rinses. If counterflow rinsing can’t be made by gravity, this can be easily overcome by using pumps or air lifts. Also, spray rinsing above process tanks or rinse tanks can take the place of a rinse tank. Parallel rinsing uses roughly “n” times the amount of water that a counterflow rinsing arrangement uses, where “n” equals the number of rinse tanks.
A second option is to reuse rinsewater, such as counterflowing an acid pickle rinse into a cleaner rinse. Another method to reduce flow is by flow-control solenoids that are energized by either conductivity sensors or programmed to energize only when parts are in tank. Lastly, pretreated wastewater can be reused in non-critical rinses; this typically requires filtration or microfiltration to remove suspended solids.
In order to maximize the cost effectiveness and efficiency of any recycling technology, all steps must be taken to reduce the dragout of process solutions into succeeding rinse tanks. Techniques to reduce dragout include:
- Operate a process tank at minimum chemical concentration;
- Operate a process tank at maximum temperature;
- Use drain boards between process tank and rinse tanks;
- Have sufficient rinsing between process tanks to avoid cross contamination;
- Reorient parts to maximize drainage;
- Make small design changes to maximize drainage;
- Use wetting agents to improve rinsing;
- Remove work at a slower rate and rotate a barrel above water after removal; and
- Use low-dragout barrels.
Recycling Technologies
metal finishing processes do not become part of the part, metal finishers have a number of technologies to recover these materials. In fact, through a combination of technologies, it is possible that a facility can accomplish a “zero” wastewater discharge to sewer or stream and
generate only a small volume of concentrated liquid and/or solid waste. By using the following technologies, finishers can practice profitable resource recovery in several ways:
- Recover the process solution and directly add back into the process tank.
- Remove contaminants from process solutions to greatly extend solution life.
- Recover metals that can be reused or sent back to suppliers and/or reclaimers.
Evaporation is the most common, simplest and, in most cases, cost-effective form of recovery. Evaporation can provide total or partial recovery. The most common use is the “natural” evaporation from the process tank. Dragout solution recovered in the following rinses is placed back into the process tank at a rate equal to evaporation. If the evaporation is sufficiently high and there is room to add a number of recovery rinse tanks, a closed-loop system can be implemented. Even in an open-loop system, a recovery rate of more than 90 percent can be obtained.
In evaluating the effectiveness of evaporative recovery, use a similar equation as with counterflow rinsing. Instead of a rinse ratio, a recycle ratio (R) is used:
R = E/D [where E = evaporation rate (gal/hr) and D = dragout rate (gal/hr)].
In order to determine the constituent concentration in the nth recovery tank, the following equation is used:
Cn = Cp/(1+R+R2...+Rn).
Through trial and error, these two equations can be used to estimate the amount of evaporation needed.
The following equation is used to estimate percent recovery:
[1 - (1/1+R+R2...Rn)] × 100% = % recovery.
For low-temperature baths, a finisher may want to investigate the use of a dragin/dragout system where a workpiece drags the recovered solution back into the process
tank. By using a dragin/dragout system, the recycle ratio increases, since the dragout rate is added to the evaporation rate in the above equation.
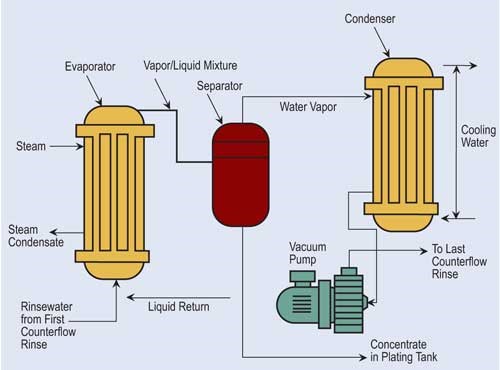
If “natural” evaporation is insufficient to provide recovery desired, finishers can turn to atmospheric and vacuum evaporators.
Atmospheric evaporators use wet surfaces, forced air and heated solution to cause evaporation.
In addition, if process solution temperature is insufficient for atmospheric evaporation, the atmospheric evaporator could be attached to an off-line heated tank. In this scenario, the counterflow from the first rinse flows to this off-line heated tank and periodically is transferred to the process tank.
While the capital costs of atmospheric evaporating systems are relatively low, they do have high operating costs for energy. It takes approximately 9,000 Btu to evaporate one gallon of water.
Vacuum evaporation lowers atmospheric pressure, reducing water’s boiling point. In a typical system, a vacuum pump draws rinse water that contains dragged-out process chemicals from selected rinse tanks through the system. One of the drawbacks for vacuum evaporation is its high capital costs. However, it has found a niche in evaporating process solutions that include heat-sensitive compounds.
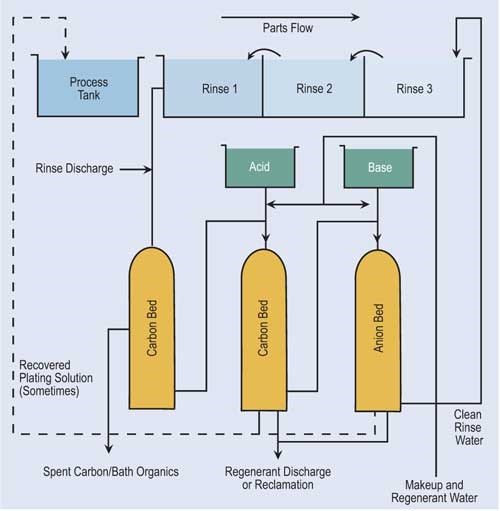
Ion exchange technology has become popular in the finishing industry. Because of major advances in resin development and technology, ion exchange is able to process a variety of chemicals and even recover selective chemicals.
In the ion exchange process, columns are packed with resin beads, which provide a large surface area for the cation and anion sites. Cationic resins exchange hydrogen ions (H+) for positive charged ions such as nickel, copper, sodium and cadmium. Anionic resins exchange hydroxyl ions (OH-) for negatively charged sulfates, chlorides and chromates. Because of the limited online time of one exchanger, two exchangers are usually used in parallel. The first exchanger operates while the second is on standby.
Regeneration of ion exchange columns depends on the type of column. If the column is cationic, acid is used. Here acid flows through the bed, exchanging its hydrogen ions for the metallic ions. Anionic columns are regenerated in a similar fashion using sodium hydroxide. Here, the sodium hydroxide flows through the bed, exchanging hydroxyl ions for the negatively charged ions.
Depending on the conditions, the regenerant solution can be directly returned to the process tank or undergo further reclamation or treatment.
Some facilities using ion exchange regenerate their own columns, while others ship them out or have someone come into the plant to do it.
A slight variation in the ion exchange technology is the use of chelating resins, which behave similarly to chelators such as EDTA. Chelating resins can hold certain transitional metal ions (such as Cu2+ or Fe3+) very tightly, but do not hold tightly lighter metals such as sodium, magnesium and calcium. Because of this characteristic, chelating resins can be used to recover low concentrations of valuable or toxic metals from solutions that contain a high level of unwanted background salts.
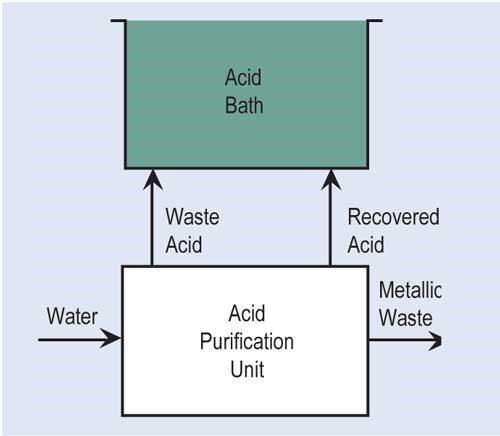
A second variation of ion exchange is a resin that can adsorb various chemicals present in solutions to varying degrees. Acid retardation is the most used adsorption technology for the recovery of acid. In this process, the waste acid is passed through a bed of special resin that has the ability to hold onto strong mineral acids such as sulfuric, hydrochloric and nitric, but not to metal salts. In order to regenerate the resin, clean water is passed through the resin bed so that the acid is desorbed from the resin.
Another variation of the ion exchange process is the affinity that biological materials, such as plant cell walls and micro-organisms, have for certain metal ions. The biological ion exchange resin is used like a conventional ion exchange resin to remove metals such as copper, iron, aluminum, cobalt, nickel, chromium, cadmium, lead, zinc, silver, gold and platinum. Unlike conventional ion exchange systems, calcium, magnesium, sodium and potassium ions don't interfere with the binding of metal ions.
Diffusion dialysis is used for the recovery of mineral acids. Used acid flows past one side of an anion membrane that allows acid to pass through to the other side where fresh DI water is passing by the membrane. The recovered acid goes back to the process bath, while rejected metals can go to waste treatment or another recovery technology.
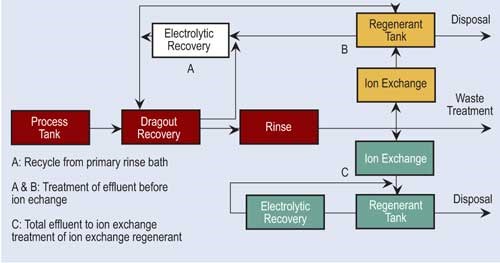
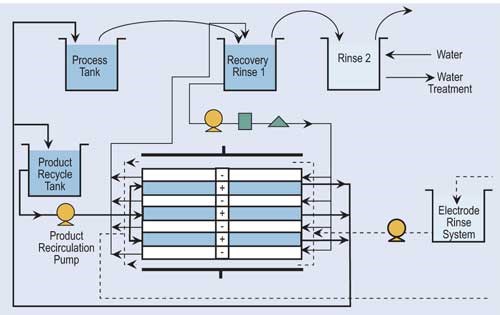
Electrowinning can remove copper, cadmium, zinc, brass, tin-lead, gold, silver and nickel from plating wastes. Historically, electrowinning needed high concentrations of metals to operate efficiently, but new technologies have lowered the minimum metal concentration of the feed waste stream.
Essentially, electrowinning is an electroplating process whereby a direct current is applied and the metal ions plate out onto a cathode. Cathodes are commonly made of polished stainless steel or carbon fibers, while anodes are usually made of non-consumable materials such as platinized titanium, ruthenized titanium, lead or graphite.
Once the cathode’s capacity is reached, it is removed from the recovery cell and the recovered metal is stripped. It may be reused on site or sold for scrap. Depending on the type of electrolytic recovery system and stripping process, the recovered metal can take the form of flakes, sheets, slurry or concentrated solution.
Often, electrowinning is combined with ion exchange to recover metals. Ion exchange processes a large volume of dilute rinse water and concentrates the metals. The regenerant flow is pumped to the recovery cell. Electrowinning can recover 90–95 percent of the available metals.
Electrowinning has proven itself to be a cost-effective and technologically sound alternative for the recovery of metals from wastes and rinse water. Electrowinning concentrates the heavy metals to their smallest volume as a metallic solid, which can be used in the plating tank or sold as scrap metal. This is important when one considers the complexities and liabilities of hazardous waste regulations.
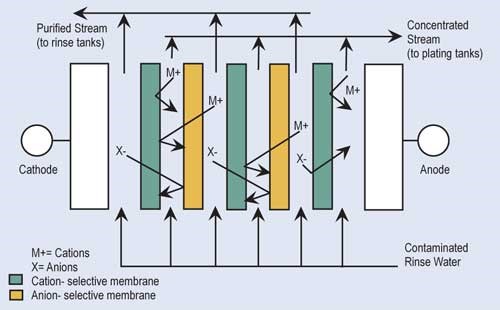
Electrodialysis (ED) is a proven recovery technology that uses membranes and direct electrical current to concentrate and separate ionic contaminants found in rinse water. In ED, the rinse water is passed through a series of alternately placed cation- and anion-permeable membranes across which there is an electropotential charge.
The cationic exchange membranes allow only the positively charged ions—such as nickel, copper and zinc—to pass through. Anionic exchange membranes allow only negatively charged ions—such as sulfate, chloride or cyanide—to pass through. Because of the alternating arrangement and electropotential, a concentrated stream and purified stream are produced. The concentrated solution is returned to the plating tank while the purified stream is returned to the rinse tank. ED units require turbulent flow and remove only 20–30 percent of the metals in solution at each pass. Therefore, the ED process is not suitable for a once-flow-through arrangement.
A variation of the electrodialysis process that is quite popular for processes using chromic acid is an electrolytic purification process that uses a semi-permeable membrane surrounding a cathode or anode. If one is trying to recover chromic acid from rinse water, the rinse water is pumped through a unit that has a semi-permeable membrane surrounding the cathode (positive charge).
The semi-permeable membranes allow the negatively charged chromate ions to be collected around the anode.
In order to purify chromic acid solutions, the solution is passed through a unit where the anode is surrounded by a semi-permeable membrane. Here, positively charged contaminants such as trivalent chromium, aluminum and iron are concentrated around a negatively charged anode and periodically removed.
ED technology has been successfully applied to a number of acid-, alkaline- and cyanide-based plating solutions, but has been most successful in recovering chromates, tin, nickel, gold and silver. Also, ED has been effective as a “roughing” treatment before reverse osmosis or ion exchange for water with a very high total dissolved solids content.
Reverse osmosis (RO) is a process that separates water from larger-molecular-weight compounds. RO accomplishes this separation by forcing the waste stream, under high pressure, against a semi-permeable membrane. This high pressure overcomes the natural osmotic pressure forcing the water to flow from a more concentrated solution to a less concentrated solution. Depending upon the chemicals to be recovered by RO, feed pressures can range from 100 to 600 psi.
RO splits the flow into two streams: con-centrate and permeate. For the vast majority of processes, the RO unit is used with recovery rinse tanks, where the concentrate is returned to the plating tank while the permeate is recirculated back through the rinse tanks. Recovery rates for typical finishing operations are approximately 95 percent or higher.
RO systems have been used to recover chromium, copper, zinc, brass, cadmium, tin, palladium and other precious metals, but the most common application for RO technology is in the recovery of acid nickel.
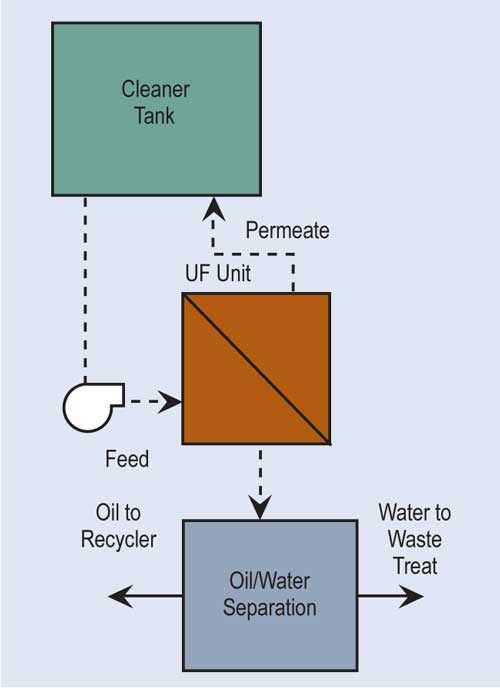
Ultrafiltration (UF) is similar to RO. It uses a semi-permeable membrane to separate, under pressure, molecular and/or colloidal materials dissolved or suspended in a liquid. But, the membrane size for ultrafiltration is approximately 20 angstroms as compared with five angstroms for most RO membranes, and its feed pressures are not nearly as high.
The most common use for ultrafiltration is the removal of oil from process solutions, particularly alkaline cleaners. This extends solution life, minimizing chemical costs and generation of wastewater treatment sludges. UF allows most, if not all, of the detergents and other chemicals in the cleaner solution to pass through the membrane for return to the process. Ultrafiltration is also used to re-concentrate paint rinsed from parts in electrocoating.
With recent advances in membrane manufacturing, cost has significantly been reduced, making it a more feasible technology.
Over the last 30 years, numerous facilities around North America have been constructed to provide off-site reclamation of heavy metals from various waste streams. These facilities not only use some of the recovery technologies described above but others that are cost effective on a large scale such as pyrometallurgy (high-temperature thermal reduction), hydrometallurgy (selective metal precipitation), crystallization and electrochemical heavy metal removal.
Because of the liability with land disposal of hazardous waste, off-site recovery opportunities should be explored. One can contact the state hazardous/solid waste regulatory agency to obtain the names of organizations in your region that offer reclamation services.
Waste generated by finishers, especially those containing chemicals such as metals, cyanides, acids and caustics, is an indicator of process inefficiencies. The technologies described above are tools to improve efficiency, making finishing services more valuable and competitive in today’s ever-changing global marketplace.
The secondary benefits are:
- More effective and efficient compliance with environmental regulations;
- Goodwill of neighbors and neighboring community;
- Business development for customers;
- Minimization or even removal of environmental liabilities, some of which can carry civil and criminal penalties.
Two keys in implementing recovery/recycling first to take a forward-looking and proactive approach. Second, it is important to conduct a comprehensive evaluation of
the entire facility. Waiting for a law to be passed will likely cause you to rush into the process, making hasty and wasteful decisions.
It is never too late to consider and implement recovery and recycling technologies, and you need not do it alone. Begin discussing these technologies with your suppliers and consultant, and attend seminars.
Related Content
Novel Wastewater Treatment Targets Micropollutants
Swiss wastewater treatment technology provider Oxyle specializes in advanced wastewater treatment for removal of highly persistent micropollutants such as PFAS.
Read MoreUltrafiltration Membranes, Filter Elements for Improved Industrial Water Reuse
Ultrafiltration membranes help with water reuse in a variety of applications.
Read MoreNASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 6th Quarterly Report
The NASF-AESF Foundation Research Board has selected a project on addressing the problem of PFAS and related chemicals in plating wastewater streams, studying PFAS destruction via electrooxidation and electrocoagulation. This sixth quarter report covers the continued assessment of eight perfluoroalkyl acids PFAAs most commonly found in wastewaters, by electro-oxidation with a Magnéli phase Ti4O7 anode across a range of anodic potentials in solutions, exploring the reaction mechanisms. To summarize, the PFAAs start to exhibit degradation behavior when the anodic potential reaches a level where water oxidation occurs, suggesting that the hydroxyl free radicals generated via water oxidation play a role in PFAA degradation.
Read MoreZinc Phosphate: Questions and Answers
Our experts share specific questions about zinc phosphate and pretreatment
Read MoreRead Next
Saving Water, Saving Chemicals, Saving Money
Metal Seal and Products installed a water treatment system and wastewater treatment system that helped it increase production, reduce reject rates and lower its expenses for chemicals, water and hazardous waste disposal…
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More