Better Alloy Control of Alkaline Zinc Nickel Baths
The factors that influence nickel content in deposits from alkaline non-cyanide zinc nickel baths.
In the deposition of the zinc nickel layer in an alkaline zinc nickel bath, corrosion protection is related, in part, to the percentage of nickel in the deposit. Therefore, an understanding of the elements that control that percentage are helpful in constructing the most effective layer.
The automotive, agricultural equipment and other durable goods industries continue to focus heavily on zinc nickel plated deposits. This has been the catalyst for the zinc nickel plating market to have more than doubled in North America in the last five years. As zinc nickel plating chemistries have improved, the cost to operate compared to the performance benefits has become much more attractive. This trend is important especially in the automobile sector where longer warranty periods will continue to drive demand for the level of corrosion resistance provided by this deposit.
Research has found that the most influential component of the overall nickel content is control of the percentage of nickel content in the bath. Current density and temperature also play a role in the overall process. Additions of sulfate and carbonate yield increased levels of nickel to a lesser degree, but the addition of more or less caustic demonstrated little impact on the overall nickel incorporation. The investigation of these factors is crucial in the development of zinc nickel plating technologies that will continue to meet the growing demand for this deposit on automotive-related components.
Zinc nickel alloy plating may be one of the more common methods for corrosion protection these days, but this has not always been the case. Alloy plating has a rich history dating back to the early 1800s. Early records of alloy plating report the issuance of a patent in England in 1838 to George Richards Elkington and Oglethorpe Barratt, who prepared coatings of copper and zinc by immersing them into a boiling solution of zinc chloride.1
In 1869, chemist Alfred Roseleur summarily dismissed zinc nickel alloy, stating that “this application is without industrial importance because nickel costs about five times as much as copper of which it possesses all of the disadvantages, and in particular its poisonous properties.”2
Automotive Industry Demand
Investigation into zinc nickel alloy electrodeposition remained largely a source of academic interest until the early 1980s, when its role in satisfying the demand for a corrosion-resistant alloy plating for the automobile industry began to emerge.3 Due to excellent corrosion resistance and self-lubricating properties, cadmium was the metal of choice for corrosion protection applications, particularly in the fastener industry,4 but restrictions to the exposure of cadmium in the United States, Europe and Japan created a demand for a suitable substitute.5,6 In fact, the alkaline zinc nickel alloy plating process addressed two separate environmental issues including cadmium exposure and exposure to cyanide baths.7
Today, protection of steel from corrosion is big business, and 50 percent of the world’s zinc consumption is applied toward this goal. The electroplating of steel covers between 25 percent and 30 percent of total zinc production.8,9
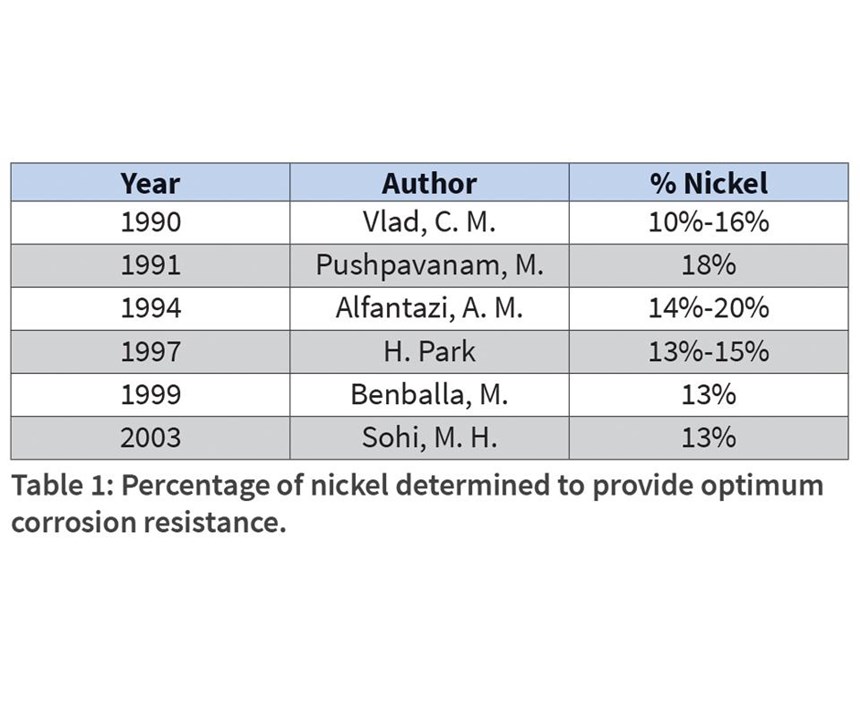
Two primary examples of zinc nickel baths prevail. Acid zinc nickel baths typically deposit nickel from 10 percent to 14 percent, whereas the alkaline baths deposit nickel from the 6-percent to 15-percent range. The correlation of nickel content (and its ideal percentage) to corrosion protection has been included in many studies, some of which are shown in Table 1. Generally, the corrosion protection of steel will increase with increasing nickel content up to about 15 percent, after which the deposit becomes more noble than the substrate, losing its sacrificial properties.
Alkaline zinc nickel baths offer a ductile deposit, corrosion resistance in salt spray (ASTM B 117) up to 3,000 hours,10 and the ability to plate in deep, low current density regions. Control over the nickel content, however, is a matter of genuine concern, since higher nickel content may, for example, lead to limited ductility (above 20-percent nickel), or difficulty passivating the deposit, which becomes impossible in alloys containing over 25-percent nickel.11 For this work, we focus on those factors that affect and control the deposition of nickel in alkaline zinc nickel baths.
Dominant Factors Influencing Ni in the Deposit
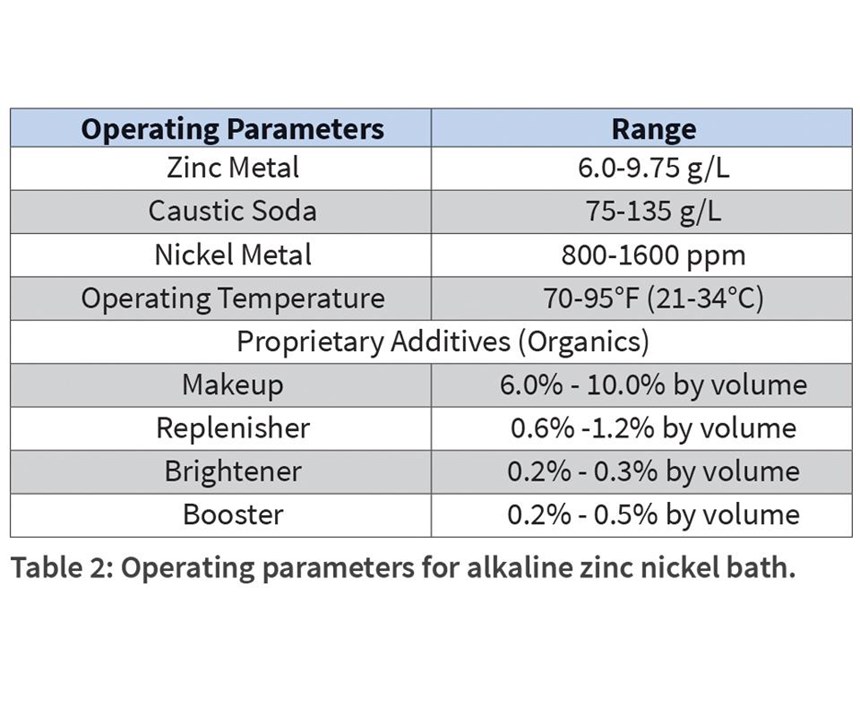
A typical alkaline zinc nickel bath may be prepared according to the components in Table 2.
Over the course of a bath’s life, components change or are depleted either through consumption or dragout. This results in change in performance of the plated parts, and requires the proper addition or makeup of replacement materials. Because there are so many variables, often all changing simultaneously, it is sometimes difficult to ascribe a performance change to a single component cause.
To track the property and material relationship in an alkaline zinc nickel bath, we prepared a model alkaline non-cyanide plating process. Zinc-nickel alloy thickness and percent-nickel composition in the deposit were measured against nickel concentration in the bath, current density, bath temperature, sulfate, caustic and carbonate load. Samples were prepared on steel Hull cell panels and plated at 2 amps for 10 minutes for comparison. Metal contents and percentages are determined by X-ray fluorescence.
Baths are prepared according to the guidance in Table 2, except for the experimental variable. Percentage of nickel content in the alloy is a valuable metric in the determination of corrosion resistance, so control of this parameter is important. Generally, it is agreed that values between 12 percent and 15 percent provide optimum protection, with a drop in corrosion resistance in deposits greater than 15 percent.12
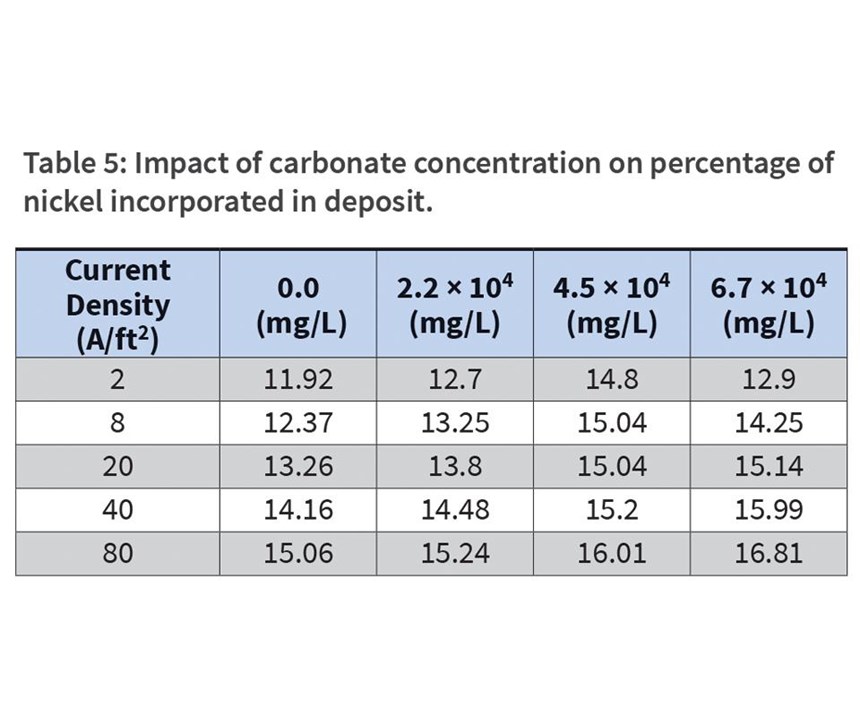
It is little surprise that increased nickel content in the bath results in increased nickel content in the deposit (see Figure 1). Similar values were obtained in a study by Hwa Young Lee and Sung Gyu Kim,13 who measured the effect of nickel concentration on nickel content in an alkaline bath at 80°F. Lee further identified the profile of the nickel deposition to be 85-percent nickel within the first 0.5 seconds of deposition, and then constant at a rate of 10 percent through the remainder of the deposit. Baths that fall below a threshold of 500-ppm nickel are unable to plate into the desired 12-percent to 15-percent goal in the bulk, regardless of current density. Baths prepared at 850-ppm nickel, however, deposit the desired percent-nickel composition at all current densities, while nickel-rich baths (1,500-ppm nickel and above) plate at target concentrations at lower current densities, but exceed the target as current densities increase. Therefore, to meet the target of between 12-percent and 15-percent nickel, it would be necessary to maintain the nickel concentration at 850 mg/L at a current density of between 20 and 60 A/ft2.
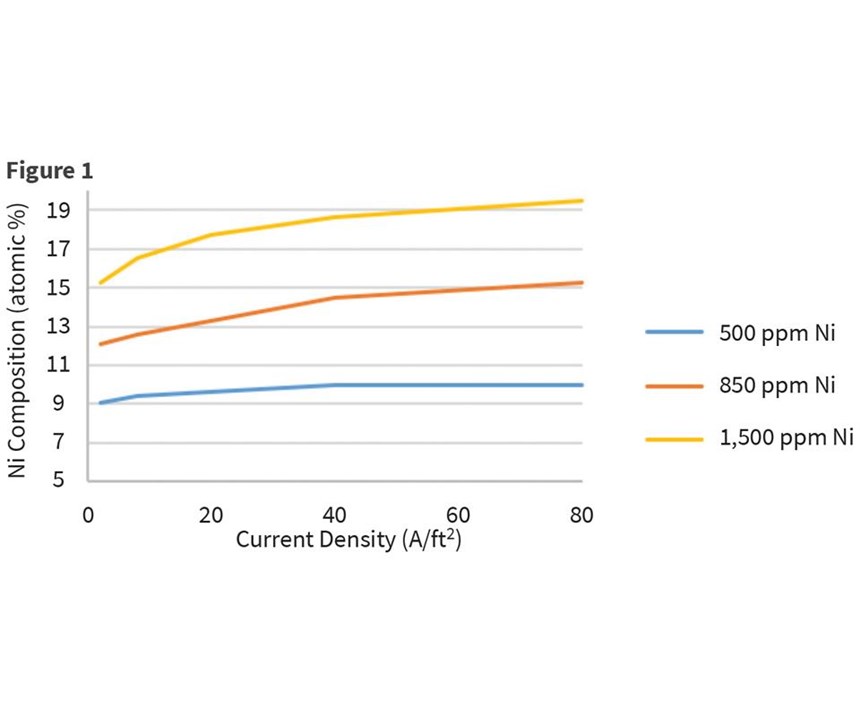
Another variable investigated was the impact of temperature on incorporation of nickel in the deposit. For a bath consisting of 7,500-ppm zinc, 850-ppm nickel, and 10-percent proprietary organics, bath temperatures varied from 50°F to 100°F. The panel was plated at 2 amps for 10 minutes and measurements were taken at 2, 8, 20, 40 and 80 A/ft2. Some minor influence of nickel incorporation was observed with temperatures over the range of current densities. Although our experiments were not performed above 80°F (27°C), Lee also reports a slight rise in nickel content with increasing temperature from 77°F through 122°F, but then a sharp increase from 140°F and higher. This was ascribed to the redissolution of zinc deposited by the alkali at elevated temperature. Results are shown in Figure 2.
Effect of Caustic, Sulfate and Other Factors
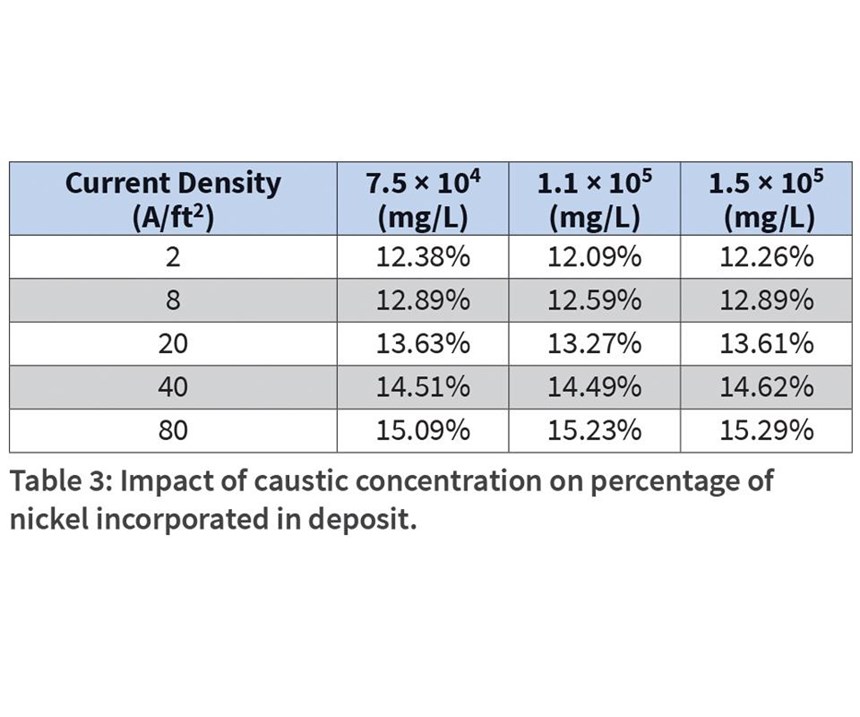
The percent of nickel in the deposit was evaluated as a function of caustic, sulfate and carbonate concentration. While the pH of the alkaline bath is very high, the effect of caustic concentration (as 50-percent NaOH) on nickel incorporation was studied (see Table 3). The optimum prescribed concentration for caustic in the bath is 112 g/L. Steel panels were run from Hull cells containing 75 g/L, and 150 g/L caustic to observe the low and high range on performance. While nickel incorporation increased as a function of applied current density, very little impact on percentage of nickel in the deposit was noticed as a result of caustic concentration.
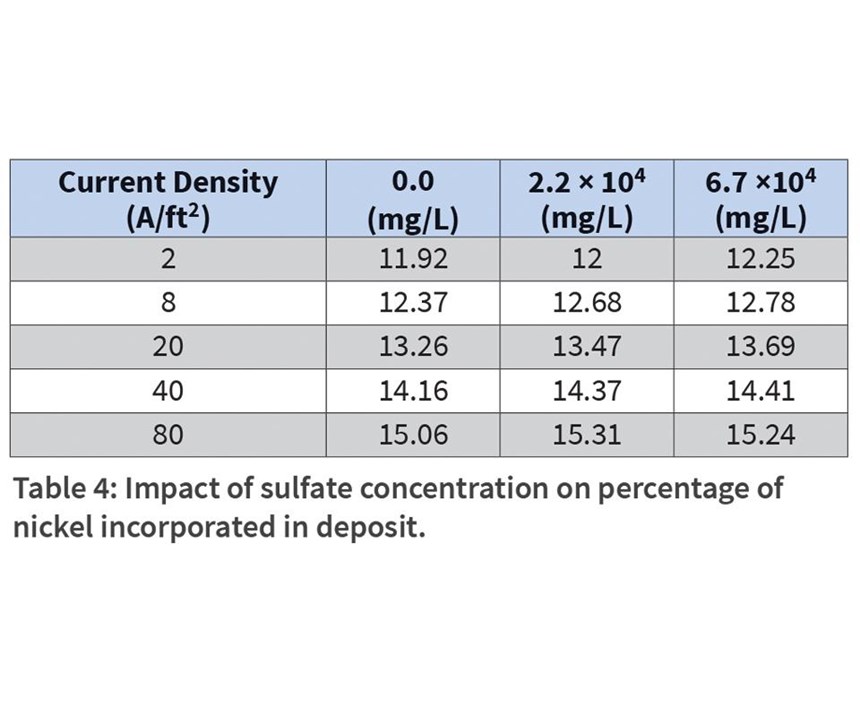
Sulfate buildup occurs as the nickel plates over time, leaving the residual anion. To replicate the buildup of sulfates in the bath, additions of sodium sulfate were added to the bath as 0 mg/L, 2.25 × 104 mg/L, and 6.74 × 104 mg/L. The amount of nickel in the plating deposit varied only slightly from sample to sample, and increased slightly as current density increased (Table 4).
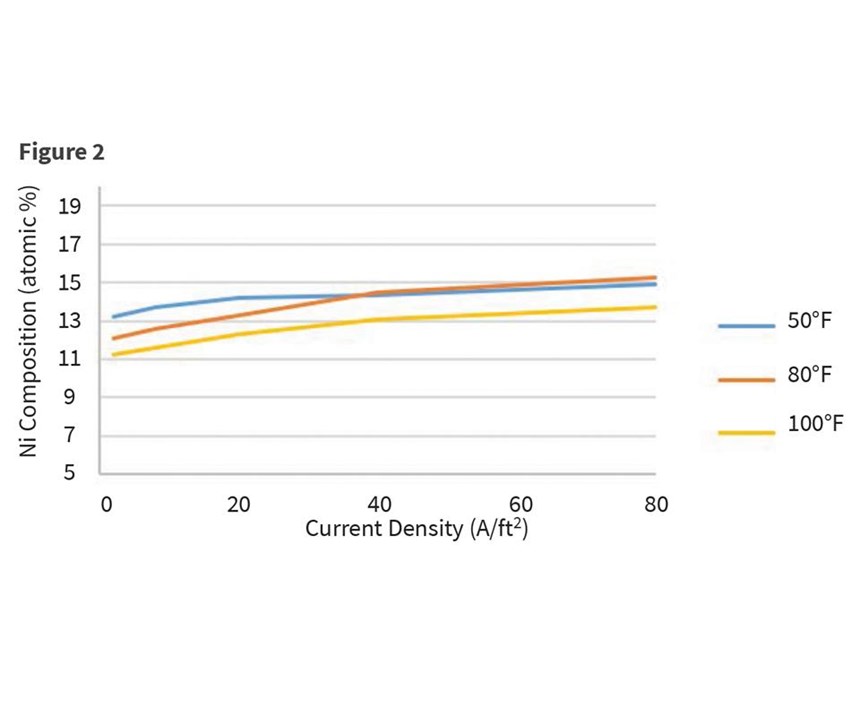
Finally, high concentrations of carbonates may slow plating speeds or lead to poor cathode efficiency. Carbon dioxide reacts with water to create carbonic acid, H2CO3, which then reacts with the alkaline solution to form carbonates. To a smaller degree, oxygen formed at the anode may react with organic materials intentionally introduced to the bath as additives. Excessive agitation and high current densities also contribute to the development of carbonate formation. Concentrations of carbonates above 75 g/L can quickly reduce plating speed. However, their impact on nickel incorporation into the deposit is found to be minimal (see Table 5). The percentage of nickel increases slightly at every measured current density, and as current densities increase within a single carbonate concentration experiment.
The factors influencing the incorporation of nickel into an alkaline-cyanide-free zinc nickel deposit were observed under controlled conditions. The single most significant influence of the nickel incorporation into the deposit was the concentration of nickel in the plating bath. In all cases, an increase in current density resulted in an increase of nickel percentage in the deposit. Increased nickel was also observed relative to temperature, sulfate, and carbonates, but to a smaller degree. Within the range of caustic applied for this work, no significant changes in nickel incorporation were observed.
Dr. Lawrence Seger, Mark Schario, Laura Bonney, Michael Liu and Rick Livingston are with Columbia Chemical Corp. For information, please visit columbiachemical.com.
Sources:
1. G.R. Elkington and O.W. Barratt, Coating Metals with Zinc etc., British Patent 7, 742 (1838)
2. A. Roseleur, Guide Pratique Du Doreur, de l’argenteur et du galvanoplaste, 2nd ed. Paris (1866)
3. K. Horikawa, J. Metal Finishing Soc., Vol 33, 360, (1982)
4. E. Groshart, Metal Finishing, (1997), 95, 79
5. M.D. Thomson, Trans IMF (Bull), 74, (3), 3, (1996)
6. J. Dini, H. Johnson, Met. Finishing, 77, (8), 31, (1979)
7. J. A. Bates, Comparison of Alkaline Zn-Ni & Acid Zn-Ni As A Replacement Coating For Cadmium, Plating and Surface Finishing, April, (1994)
8. T. Asamura, Proceedings 4th Int. Conf. on Zinc and Zinc Alloy Coated Steel Sheet, (Galvatech, 98), (1998), Chiba, Japan, The Iron and Steel Institute of Japan pp. 14-21
9. F. E. Goodwin, Proceedings 4th Int. Conf on Zinc and Zinc Alloy Coated Steel Sheet (Galvatech 98), (1998), Chiba, Japan, The Iron and Steel Institute of Japan, pp.31-39
10. E.W. Brooman, Metal Finishing, (2000), 98, 42.
11. M. Pushpavanam, S.R. Natarajan, K. Balakrishnan, L.R. Sharma, Journal of Applied Electrochemistry, (1991), 21, 642.
12. P. Viera, B. Van den Bossche, A. Rose, Characterisation of a Zinc/Ni Plating Bath, Elsyca, (2009), 9, 3.
13. H. Y. Lee, S. G. Kim, Characteristics of Nickel Deposition in Alkaline Bath for Zn-Ni Alloy Deposition on Steel Plates, Surface Coatings and Technologies, 135, (2000), 69-74.
Related Content
How to Choose Between Sulfate and Chloride-Based Trivalent Chromium
There are several factors to consider when choosing between sulfate and chloride-based baths for trivalent chromium plating. Mark Schario of Columbia Chemical discusses the differences and what platers should keep in mind when evaluating options.
Read MoreHow to Maximize Nickel Plating Performance
The advantages of boric acid-free nickel plating include allowing manufacturers who utilize nickel plating to keep up the ever-changing regulatory policies and support sustainability efforts.
Read MoreTop Reasons to Switch to a Better Cleaning Fluid
Venesia Hurtubise from MicroCare says switching to the new modern cleaning fluids will have a positive impact on your cleaning process.
Read MoreAlkaline Cleaning Guide
Gregg Sanko, Senior Chemist, Oakite Products, Inc. provides an overview of the alkaline cleaning process.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More













.jpg;maxWidth=300;quality=90)








