Catalytic Oxidizers and Title V Requirements
It is important to understand the various aspects of catalytic oxidation and consider options such as monitoring inlet/outlet temperatures, T across the catalyst, periodic laboratory testing of catalyst samples and preventive maintenance procedures as means of assuring continuous compliance…
Federal and state environmental regulations are requiring higher destruction and improved capture of volatile organic compounds (VOCs). Title V requirements may also increase the need for end users of emission control equipment to demonstrate continuous compliance. With many state and local permitting authorities issuing Title V permits, the subject of periodic monitoring has proven to be one of the most challenging aspects of the rule. This portion of the rule, Title 40 of the Code of Federal Regulations (40 CFR), part 70, has been one where there have been many interpretations of this requirement. Catalytic oxidizers have long been used as reliable, cost-effective solutions for VOC destruction. However, since catalytic oxidation is not an area of expertise of the majority of emission control equipment users, there have been various questions and concerns voiced about the longevity of the catalyst. In order to best address this subject, it is wise to review how this technology works and how periodic monitoring under the requirements of Title V can be effectively achieved.
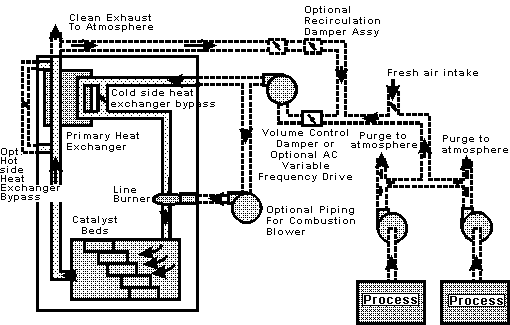
Catalytic oxidizers can convert VOCs to carbon dioxide and water at much lower temperatures than thermal oxidizers by using a catalyst inside the combustion chamber. This significantly reduces the need for auxiliary fuel, resulting in lower operating costs. A flow schematic for a catalytic oxidizer is shown in Figure 1 below. The basic elements are a pre?heating section that is used to heat the process gas, followed by the catalyst bed and heat recovery equipment. Catalyst systems operate between 400-70°F. Various types of catalyst are available in the marketplace.
A typical catalyst is composed of
- Active catalytic material - a chemical that provides the catalytically active sites for the desired reaction. The most active materials for oxidation of VOCs are noble metals (Pt, Pd) and certain metal oxides. Most commercial catalysts contain noble metals.
- Support material - usually an inert, highly porous metal oxide (alumina, silica or titania) with large specific surface (BET surface areas can be between 150-300 m2/g of support). The catalytically active material is dispersed over the internal surface area of the support, providing a display surface for the active metal sites.
- Mechanical support - provides the physical structure and mechanical integrity of the catalyst body. It can support material that has been shaped (alumina spheres) or a different material and structure (metal foils, ceramic monoliths) over which the catalyst is applied.
Catalysts are used in two common configurations:
- Packed beds - loose, bulk materials packed into a container and retained by perforated plates. The exhaust gases flow through the catalyst bed, providing mixing and contact between gas and solid. Typical packed bed catalysts include bead catalysts.
- Monolith catalysts - ceramic or metal honeycombs that provide support for the catalytically active material. The exhaust gases flow through the individual channels of the monolith. Although the contact between gas and solid is not as efficient as in packed beds and the poisoning resistance is lower than the bead?type catalyst, monolith systems can afford a low pressure drop.
The basic catalytic system includes a fan to pull the air from the process and push it through the oxidizer, a heat exchanger to preheat the exhaust stream to the oxidizer, a burner to heat the air stream up to catalyst activation temperature and a catalyst bed to hold the catalyst in place.
EPA provides a number of guidance documents for periodic monitoring. For example, Section 504 of the Clean Air Act (Act) makes it clear that each Title V permit must include "conditions as are necessary to assure compliance with applicable requirements of the Act, including the requirements of the applicable implementation plan" and "inspection, entry, monitoring, compliance certification and reporting requirements to assure compliance with the permit terms and conditions."
As EPA further address the subject of periodic monitoring, it notes that this must provide a reasonable assurance of compliance with requirements applicable to the source. EPA states, "The periodic monitoring process should begin by evaluating whether monitoring, including record keeping, reporting or periodic testing, applies to the emissions unit in question under existing applicable requirements for that unit. If the already?required monitoring is sufficient to yield reliable data from the relevant time period and is representative of the source's compliance with a particular applicable requirement, then no further monitoring for that applicable requirement at that emission unit is required in the permit."
This leads us to determine what data can provide proper assurance of compliance during operation. According to EPA, "Operational data collected during performance testing is a key element in establishing indicator ranges; however, other relevant information in establishing indicator ranges would be engineering assessments, historical data and vendor data. The permit should also include some means of periodically verifying the continuing validity of the parameter ranges."
This provides the guidance that allows us to determine the data to show assurance of compliance during operation. In many cases, it has been assumed that an ideal indicator of compliance for a catalytic oxidizer is the temperature increase across the catalyst bed (DT), which does provide an indication of the heat release from the VOCs converted in the process. Unfortunately, this is an ideal indicator only for those applications where the VOC concentration is consistent. Many applications have varying VOC loading as process operational parameters vary. This situation becomes further complex when more than one process is controlled by a single emission control system. Here the potential for variation in DT is even greater and using such an indicator would prove to be misleading for anyone using DT for compliance purposes.
This situation has been addressed by EPA in its Compliance Assurance Monitoring rule where it states that "other information such as historical monitoring data and engineering assessments can be used in combination with parameter data collected during performance testing to establish indicator ranges that are representative of normal operating conditions. As long as changes are not made to the control device settings used during normal operation (e.g., changes to oxidizer temperature set points), the results of performance tests can be used in combination with historical monitored data collected during periods of normal operation and engineering assessments to establish indicator ranges indicative of normal operation."
A catalyst can lose its activity by loss of surface area due to sintering (exposure to very high temperatures) or poisoning/masking by compounds such as silicone and phosphorous. To ensure that a catalytic oxidizer is meeting VOC conversion requirements, it is important that catalyst deactivation is measured and procedures are put in place to continually monitor the performance of the catalyst. There have been some issues and concerns about the best possible method of field catalyst sampling so that a representative sample could be obtained.
The single most important factor in any analysis technique is the collection of an accurate sample. If a non?representative sample is taken, the results may be skewed. Any recommendation based on these results would be biased. In most catalytic systems, air flow moves vertically from top to the bottom or horizontally from one side to the other. As the leading edge of the bed is exposed to the process gas, any poisons in the process gas will initially deposit there. Therefore a sample taken from this part of the bed would be expected to exhibit "worst case" performance. Conversely, a sample taken from the trailing edge of the bed would be expected to exhibit "best case" performance.
In order to make a good recommendation about the status of the catalyst bed, it is important to determine the condition of the catalyst at the top, middle and bottom of the bed. Depending on the amount of catalyst in the unit, only a portion of the catalyst bed needs to be in good condition in order to meet expected clean?up levels.
A variety of sampling techniques can be used to obtain a meaningful catalyst sample. Many oxidizers contain sample cores that can simply be extracted from the catalyst bed and labeled "TOP" and "BOTTOM." The core is split into various parts in the lab to test catalyst performance at various levels. Monolith cores can be tested as is or by extracting smaller samples from the larger cores.
Typically, bead samples that arrive in sample cores are tested in two segments. The top part of the core is tested first. If it appears to be in good condition, no further testing is required. As was explained earlier, these are "worst case" beads, and, if they are in good condition, the rest of the bed can be assumed to be in better condition. However, if the beads do not appear to be in good condition, a second test is done on the beads in the middle of the core. A recommendation is then made based on those results. Samples also can be tested as a composite of the whole bed.
If an oxidizer does not contain sample cores, a different approach is recommended. Most bead-based oxidizers contain their catalyst in trays. Several trays are used (see Figure 2) to minimize total pressure drop. Since each tray is exposed to the same air stream, it is necessary to take samples from only one tray or bed. Three samples are taken from this one bed and put into three separate clean containers. The first sample would be catalyst from the top 1-inch of the bed and is labeled "TOP." Without disturbing any more of the bed than is necessary, the top catalyst is moved away so that a sample of the middle can be taken. This sample is labeled "MIDDLE." Continue to carefully move catalyst away from the sample area until the bottom inch of catalyst is exposed. This third sample is taken from the last inch of the catalyst bed. It is labeled "BOTTOM." The three portions are sent in for testing in their separate containers. This approach is less rigorous than the sample core approach.
Catalyst samples brought to the laboratory are tested for VOC destruction efficiency, contamination (Si, P, Cl, etc.) and BET surface area. Conversion efficiencies obtained at various temperatures in the laboratory accurately reflect the relative activities of the samples tested compared to fresh catalyst and compared to other catalyst in varying levels of degradation. But, it doesn't equal the conversion efficiency to be expected from a unit containing this catalyst. This is due to different process conditions in the laboratory versus the field, wall effects in lab units and so on. Laboratory testing provides activity levels under standard conditions, which allow the sample to be compared to a large data base of samples tested under those same conditions. These standard conditions may not be the same as field conditions. Since process conditions in the field can vary considerably, this type of standardized testing ensures an apples-to-apples comparison and allows a better judgment call when deciding whether catalyst replacement should be considered.
BET surface area is also a measure of the performance of the catalyst. As the catalyst is exposed to high temperatures, it can sinter and lose its surface area, which results in loss of catalyst activity. A loss in catalyst surface area of more than 30% could result in substantial loss in the activity of catalyst in the field.
Another indicator of catalyst life is the amount of poison or masking agents on the catalyst. Typically, in a bead catalyst, if there is more than 1.5% of the combination of silicon and phosphorous, the catalyst activity may be low and not sufficient to meet field requirements. A catalyst supplier usually makes a recommendation as to whether the catalyst is in good condition, poor condition or borderline condition. If the catalyst is in borderline or poor condition, it is generally recommended that a stack test be performed.
It is difficult to predict the life of a catalyst. It varies depending on the operational history of the field unit, the catalyst composition, the susceptibility to poisons and so forth. However, most catalyst manufacturers can compare a sample's laboratory performance with tests that have been performed in the past and determine whether there is likely to be a problem or not. While the actual field cleanup of the unit can't be predicted, a catalyst that performs well by lab standards will perform well in a unit, provided the unit is mechanically sound. Therefore, it is highly recommended that regular preventive maintenance checks be conducted. Along with regular catalyst testing, this is an excellent and cost-effective way to be sure that oxidizer performance expectations are met.
The best method of assuring compliance of a catalytic oxidizer is to use a combination of factors:
- Use the catalyst activation temperature determined in the compliance test to set the minimum operating temperature, which should be recorded to show one parameter of compliance.
- Submit the catalyst for testing based on the manufacturer's recommended time frame, which is based on historical engineering data on performance within the specific industrial sector.
- Have the oxidizer inspected in accordance with the manufacturer's recommendations or by the manufacture's representative to provide assurance of operational requirements being maintained.
Catalytic oxidizers have been proven to meet BACT and LAER requirements in many industries. The lower energy use provides an added advantage as precious energy resources are not consumed in the process of achieving air quality goals. With the development of new and improved catalysts and properly designed systems, the ability to prove compliance via periodic monitoring can be achieved in the steps noted above.
Related Content
Ultrafiltration Membranes, Filter Elements for Improved Industrial Water Reuse
Ultrafiltration membranes help with water reuse in a variety of applications.
Read MoreZinc Phosphate: Questions and Answers
Our experts share specific questions about zinc phosphate and pretreatment
Read MoreNASF/AESF Foundation Research Project #121: Development of a Sustainability Metrics System and a Technical Solution Method for Sustainable Metal Finishing - 15th Quarterly Report
This NASF-AESF Foundation research project report covers the twelfth quarter of project work (October-December 2023) at Wayne State University in Detroit. In this period, our main effort focused on the development of a set of Digital Twins (DTs) using the Physics-Informed Neural Network (PINN) technology with application on parts rinsing simulation.
Read MoreNASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 12th Quarterly Report
This NASF-AESF Foundation research project report covers the 12th quarter of project work (October – December 2023) at the University of Georgia. In our previous report, we described our work on performance and effect of surface fluorinated Ti4O7 anodes on PFAS degradation in reactive electrochemical membrane (REM) mode. This quarter, our experiments involved utilizing porous Ti4O7 plates serving both as anodes and membranes. Tests compared pristine and F-18.6 Ti4O7 anodes at current densities of 10 mA/cm2 and 40 mA/cm2. This 12th quarterly report discusses the mechanisms of the effects on EO performance by anode surface fluorination.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More














.jpg;maxWidth=300;quality=90)







