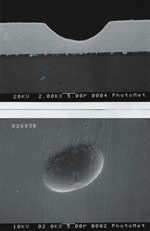
Pitting on Thick Electroless Nickel Deposits
Plating thick EN deposits without pitting
Many applications today require electroless nickel (EN) deposits with flawless uniformity—applications in the disk and connector industries, for example. Parts destined for application in the aerospace, automotive and oil & gas industries can also benefit from uniform, pit-free EN deposits.
But pitting, especially in thicker deposits, has plagued EN platers for many years. Process variables such as filtration, agitation, substrate preparation, and nickel chemistry all have an impact on deposit integrity. Having a better understanding of the mechanisms which cause pitting, and thereby having an opportunity to minimize them, will not only increase the number of future applications for electroless nickel but will also lead to other improvements of EN coatings such as better corrosion resistance, wear resistance, solderability and even stain resistance.
Much research has been devoted to the understanding of porosity of EN coatings, because the presence of pits upsets the structural homogeneity of the deposit and is usually the cause of corrosion or chemical attack of the coating-substrate combination. Pits in EN coatings can exist as relatively large (>2 μm) defects, or might manifest themselves microscopically (<0.1 μm). This latter type of pitting can have a deleterious effect on properties such as stain resistance, solderability or even the magnetic properties of the EN deposit.
EN coatings are produced by a delicate balance of competing chemical reactions located at the surface of the substrate to create a nickel/phosphorus matrix that can be either crystalline or amorphous. These chemical reactions are dependent on surface reactions, which can be divided into the following five elementary steps:
- Diffusion of reactants to the surface
- Absorption of reactants at the surface
- Chemical reaction at the surface
- Desorption of products from the surface
- Diffusion of products away from the surface.
These are consecutive steps, and if any one of them is disrupted surface anomalies may occur that can manifest themselves as pits. These deposit anomalies can be classified into five distinct categories: Particle, gas, organic, heavy metal and substrate. The presence of each type of pit can be created by a certain combination of conditions, which this article will address.
Particle Pitting
Particle pitting is one of the most common sources of pitting in electroless nickel. Typically, thick EN deposits (> 1 mm thickness) are most susceptible to this type of pitting due to the long immersion times required.
Particle pits are generally irregular in shape and can vary in size from less than 0.1 to greater than 2 μm in size. Generally, particle pits manifest themselves in shelf areas of the substrates.
There are two fundamental sources of particles that contribute to particle pitting: Particles formed in solution and particles of extraneous origin. Particles that form in solution can be created by the formation of small gelatinous precipitates commonly referred to as colloids. These are created by either nickel phosphite generated in the bath or production of metal hydroxides resulting from pH adjustments made to the EN chemistry.
Nickel phosphite is created as a byproduct of the chemical nickel reduction process. Its presence is dependent on bath pH, age of the chemistry, and the type of complexors present in the EN formulation.
Metal hydroxides can be a combination of nickel, iron, and zinc hydroxides. They are created by pH adjustments made to the EN chemistry. Depending on use of a weak base or strong base for pH adjustment, a metallic hydroxide can form and create a gelatinous hydroxide precipitate.
Both of these types of colloidal particles affect the chemical activity in the diffusion zone mentioned earlier. Research has shown that the addition of certain dispersants can reduce the deleterious effects of colloidal formation in some applications.
The second type of particle that can cause pitting comes from extraneous origins. These particles are introduced as a result of poor rinsing, poor cleaning, and/or poor housekeeping.
Sources of foreign particles are many. Drag-in from cleaners, zincates, and acid pickles is very common. Cleaners can bring in silicates, phosphates and carbonates which can be insoluble in EN baths. Zincates can introduce metallic hydroxides, while acid pickles can add iron salts.
Unfiltered air can bring inert particles into the bath, which not only cause deposit irregularities but also nodulation to the EN deposit. The combination of these multiple sources can lead to catastrophic pitting.
Good filtration is usually the best line of defense against particle pitting. Filtration at the 1-μm or submicron level is preferred, especially for high-tech applications and thick EN deposits. Complete bath transfer of the working bath through a filter bag before plating is always a good idea to ensure particle-free EN performance.
Ways to mitigate particle pitting include:
- Filter continuously at 1 μm or less
- Reduce drag-in of pretreatment chemicals
- Monitor orthophosphite build-up
- Eliminate outside sources of foreign particles
-
Add dispersant agents.
Gas Pitting
Pits due to gas bubbles are a common occurrence. These defects are usually circular in appearance and can vary in size. Depending on the surface tension of the solution and/or the concentrations of the constituents of the EN chemistry, this type of pitting can be of the same magnitude as particle pitting. But when gas bubbles cling or reform in the same location on the part, the pits can be fairly large and actually noticeable by the naked eye. Typically, this type of pitting can be reduced in several ways, including:
- Improving solution agitation
- Using work-rod agitation
- Adding surfactants.
Solution agitation plays a large role in reducing this type of pitting. Although agitation of the EN bath or movement of the parts is not absolutely necessary, it is usually advisable. The hydrogen gas evolution at the catalytic surface is removed more efficiently as a result of such agitation or movement.
Deposition rate is also improved in acid EN chemistries utilizing work movement. This may be explained in terms of removing the gas bubbles at the surface and raising the pH within the diffusion zone. It has been reported that increasing the flow rate of the EN solution past the parts being plated in solution created a smoother deposit, and that the plating process will produce acceptable coatings when the velocity of the solution is less than 4 ft/sec.
According to some researchers, the maximum value for introduction of air into the solution is 300 mL/sec/L. In my opinion solution movement by sparging techniques is superior to air agitation. Recent tests confirm that today’s newer and more stable EN chemistries actually pit more with the use of air agitation.
Finally, additions of a new generation of surfactants have proved useful in reducing gas pitting in some high-tech applications. Lowering surface tension and reducing bubble size did reduce gas pitting.
Organic Pitting
Organic pitting is usually created by the presence of oil or pretreatment chemicals. This type of pitting usually occurs in clusters and is flat-bottomed in appearance. Plating rate suppression is also a common occurrence with this type of contamination. The most common sources of organic contamination are drag-in of pretreatment chemistry, oil from unfiltered air, lubricants used in plating equipment, masking agents and plasticizers from hoses or liners.
Drag-in of machine oil or rust preventative compounds is typical in some plating operations. Displacement cleaners often allow oils to float to the surface of certain cleaning solutions and thereby allow racks or barrels to drag oils down plating lines. For that reason, emulsifiable cleaners are highly recommended.
Acid pickles with black rings around the top of their tanks are another sign of oil contamination. Sulfur odor is another typical sign of contamination in acid cleaners/pickles. Air blowers can also be a source of oil, which can find its way into the plating solution. Filters placed after these blowers can prevent unwanted oil from this source. Carbon filtration has been used in EN baths to remove some organic contamination, but with limited success.
Good housekeeping is always necessary to avoid organic drag-in from sources such as overhead hoists, contact saddles on the tanks or lubricants used on plating barrels. Preventive maintenance will certainly help to circumvent organic contamination, and ways to do this include:
- Replacing cleaners regularly
- Monitoring lubricants used in equipment
- Monitoring air supply
-
Providing good rinsing techniques.
Heavy Metal Pitting
Pits due to heavy metal poisoning are also a relatively common occurrence. This type of pitting typically has an affinity for edges and usually penetrates down to the base metal. Generally, heavy metal pitting is most evident in bright, mid-phosphorus chemistries as well as some of the newly introduced RoHS-compliant chemistries.
Certain heavy metal cations used in EN are sometimes referred to as “catalytic poisons.” These metallic poisons are usually found in stabilizer classes I and III. Note also that at extremely low concentrations, these heavy metal cations can appreciably alter the catalytic activity of the EN chemistry. These ions not only suppress the kinetic energy of the reduction process, but they also poison the surface by absorbing preferentially over the nickel at the substrate surface.
Poisoning begins when the surface is filled with catalytic poison faster than nickel can be deposited. Usually, increasing the temperature of the EN chemistry will incrementally increase the speed of nickel plating and the diffusion coefficient, minimizing the poisoning effect of the heavy metal stabilizer.
Sources of heavy metal contamination include drag-in of pretreatment chemicals, base metal impurities and EN chemistry.
Heavy metals are common constituents of electrocleaners, pickles and rinse waters. Previous cleaning of stainless steel, leaded steels, and copper and its alloys can and will contaminate EN chemistries. Periodic dumping of these pretreatment chemistries and rinses is highly recommended.
Alloys such as leaded steel and brass can also pit during EN plating due to presence of their alloying constituents at the surface. By selectively removing these catalytic poisons chemically (for example, using a fluoride pickle or nickel strike), platers can dramatically reduce this source of pitting.
Finally, EN chemistries can have a profound influence on pitting due to heavy metals used as stabilizers and/or brighteners. Recent information gathered from an automotive application proved without a doubt that the presence of just 1 ppm of a certain heavy metal brightener/stabilizer increased the tendency of pitting of the EN chemistry on the company’s parts. In general, avoiding the presence of heavy metals reduces pitting in EN.
Substrate Pitting
Pitting due to substrate imperfections does not figure as prominently as other forms of pitting, but it does exist. Generally, substrate pits are irregular in appearance and can vary in size. Sources of this type of pitting include substrate imperfections and overly aggressive pretreatment.
Substrate imperfections can vary from mere scratches to corrosion sites that were previously removed from the substrate (i.e. rust). Because electroless nickel plates uniformly, any pitted surfaces entering the EN bath will appear pitted after plating. Unfortunately, EN does not level like acid copper, and the filling-in of these voids is not possible. Bright dipping of steel and copper parts before EN plating has helped eliminate some of these smaller imperfections of the base metal, but only to a moderate degree.
Overly aggressive pretreatment can also be detrimental in terms of pitting. Degrading the substrate surface by long immersion times in an electrocleaner or acid pickle can leave the surface dull, rough and pitted as well. Substrate pitting can be identified simply by stripping the EN coating and observing if the surface imperfection is still present on the base metal part.
Related Content
Troubleshooting Alkaline Zinc
One of the most common problems that can arise when plating with alkaline zinc is an imbalance of brightener in the solution. In this helpful Ask the Expert article, Chad Murphy of Columbia Chemical discusses how different zinc metal concentrations and brightener concentrations can impact efficiency.
Read MoreAdvantages to Pumped Eductor Agitation
Not all agitation methods are created equally. Pumped agitation with eductor nozzles can improve process tanks and quickly show a reduction in operating costs while keeping staff safe, following environmental legislation and preventing pollution.
Read MoreAn Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read More3 Tests to Ensure Parts are Clean Prior to Plating
Making sure that all of the pre-processing fluids are removed prior to plating is not as simple as it seems. Rich Held of Haviland Products outlines three tests that can help verify that your parts are clean.
Read MoreRead Next
Episode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More