AES Research Project #41: Plating on Aluminum, Part 2: Morphology
Originally published in 1980, this paper was the second on AES Research Project #41, plating on aluminum, at the National Institute for Science and Technology. A study of the morphologies of zinc and tin immersion deposits on Al and its alloys showed that zinc formed epitaxial deposits on alloys, as noted on pure Al. Similarities exist between the zincate and stannate processes; in particular, the same crystallography orientations are active in both systems. Comparisons between zinc deposits produced from solutions containing ferric chloride and Rochelle salts and deposits from the same solution without these additions show that ferric chloride plays a fundamental role in the deposition process.
Immersion Coatings on Aluminum
Part 2: Morphology
by
D. S. Lashmore
Editor's Note: Originally published as D.S. Lashmore, Plating and Surface Finishing, 67 (1), 37-42 (1980), this paper was the second of several reports on AES Research Project #41, a study of the technology of plating on aluminum, at the National Bureau of Standards, now the National Institute for Science and Technology. A printable version of this paper can be accessed HERE.
ABSTRACT
During a study of the morphologies of zinc and tin immersion deposits on aluminum and its alloys, zinc was found to form epitaxial deposits on alloys, as was previously observed on pure aluminum. Similarities are shown to exist between the zincate and stannate processes; in particular, studies of deposits on single-crystal spheres show that the same crystallography orientations are active in both systems. Comparisons between zinc deposits produced from solutions containing ferric chloride and Rochelle salts and deposits from the same solution without these additions indicate that ferric chloride plays a fundamental role in the deposition process.
Introduction
In order to electroplate on aluminum, it is standard practice to first apply an immersion deposit of either zinc or tin. Of these, zinc has been the more common,1 but tin is gaining acceptance,2 particularly in the automotive industry. The function of these immersion coatings is the replacement of the natural oxide always present on aluminum, irrespective of the cleaning process, with an oxide-free metal on which adherent metallic coatings can be deposited. Of the two processes mentioned above, the zincate process better lends itself to investigation, not only because it is widely used but also because its non-proprietary simple composition facilitates the understanding and promotes the duplication of results of different investigators. As will be shown below, close parallels exist between these two processes, so that a study of one process aids the understanding of the other. The zincate process has been reviewed by a number of investigators3-7 and is described by an ASTM standard.8
In this study, two types of zincate solutions were considered. Solution I consisted of 525 g/L of sodium hydroxide and 100 g/L of zinc oxide. Solution II had, in addition, 1.0 g/L of ferric chloride and 10 g/L of Rochelle salts. The overall reaction which takes place in Solution I can be represented by:
(1) 3Na2ZnO2 + 2Al + 2H2O ⇒ 2NaAlO2 + 3Zn + 4NaOH
The actual form of the zincate ion in solution9 is probably Zn(OH)4-2, so that the specific reactions can be written as:
(2) Anodic: Al + 3OH- ⇒ Al(OH)3 + 3e-
Al(OH)3 ⇒ AlO2- + H2O + H+
Cathodic: Zn(OH)4-2 ⇒ Zn+2 + 4OH-
Zn+2 + 2e- ⇒ Zn
H+ + e- ⇒ H ⇒ 1/2 H2 (g)
Even though equation (1) has been experimentally verified only for solutions of type I,10 the implication of the literature11 is that it must also hold for solutions of type II. This is not the case, as shown by weight gain-loss measurements in Fig. 1. These measurements were made by weighing a specimen before and after zincating. stripping the zinc in a solution of 50% nitric acid, then reweighing. The specimens were prepared from metallographically polished aluminum zincated for 1 min according to the appropriate ASTM standard.8 From the slope (3.7) of the solution II curve, it can be inferred that, for every zinc atom deposited on the surface, nine aluminum atoms must have been dissolved. Therefore, it can be concluded that the overall reaction for solutions containing ferric chloride and Rochelle salt is more complex than that indicated by equation (1).
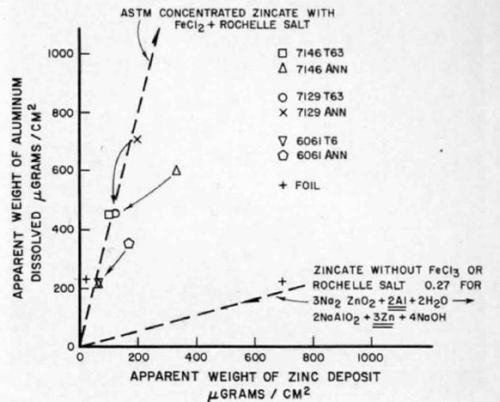
Figure 1 - Weight gain measurements of various alloys in zincate solution.
The morphology of the zinc is important. Because the adhesion of subsequently deposited layers depends critically on the nature of the interface, the structure of the zinc and its relationship to the substrate orientation was determined as a first step in this investigation. To supplement these results, further studies were undertaken on an aluminum single-crystal sphere.
Procedure
The morphology of the zinc deposits was investigated by electron microscopy of zincated, thin, aluminum foils. The 3-mm-diameter specimens were prepared by polishing 99.999% aluminum to achieve electron transparency using a twin-jet electropolishing device. The electrolyte was a 20% solution of perchloric acid and methanol cooled to 240°K (-30°C) and operated at about 22 V. Alloy specimens were prepared from spark-cut foils about 0.3-mm thick which were then thinned to electron transparency using the same procedure as with pure aluminum. The specimens were examined in a transmission electron microscope (TEM) equipped with an anticontamination stage and a double-tilt specimen holder. The zincate solution was applied to only one side of the specimen. The deposition time was 1 min unless otherwise stated.
A single-crystal sphere was prepared by appropriately machining a 25-mm single crystal of 99.99% aluminum. It then was chemically polished in a solution of 94% phosphoric acid and 6% nitric acid at 373°K until the extensive cold work induced by the machining process was removed. The perfection of the crystal was determined by etching in 15% NaOH and examining the perfection of the etch patterns and finally by taking Laue back-reflection, x-ray patterns of the principal orientations and examining the perfection of the resultant spot patterns.
Morphology on pure aluminum
A number of differences in the deposits formed from the two kinds of solutions are illustrated in Figs. 2a and b, which show TEM micrographs taken under identical conditions. The 99.999% aluminum substrates were both of the (100) orientation. In Fig. 2a, deposits grown from Solution I exhibit a number of long rectangular crystallites, a large number of extremely small crystallites and an absence of larger rectangular crystallites. As shown in Fig, 2b, deposits formed from Solution II contain no extremely small crystallites of two-fold symmetry.
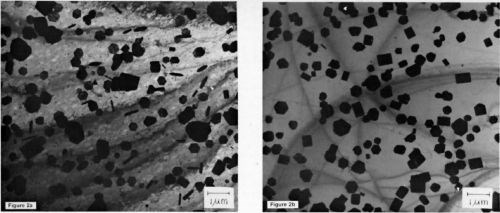
Figure 2 - Bright-field TEM micrographs comparing morphologies of zinc deposited by immersion (a) in solution 1 and (b) in solution 2 (100 keV).
Table 1 - Distribution of morphologies.
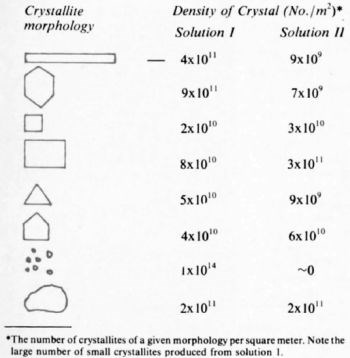
Perhaps equally important, few of the crystallites of six-fold symmetry are electron transparent, whereas many of those formed from Solution I are. implying a more rapid growth along the c-axis in deposits formed from Solution II. In deposits from both solutions, one crystal growing on another can be observed. Some of these results are summarized in the distribution of morphologies (Table I), estimated from the micrographs of Fig. 2. The large number of small crystallites observed in deposits from Solution I (1 × 1014) implies a non-continuous deposit. These small crystallites are essentially absent in deposits from Solution II.
A previous TEM study11 revealed epitaxial orientation of zinc on all of the aluminum principal planes and, moreover, that under these zinc crystallites a thin semi-continuous zinc film exists upon which the larger crystallites grow. The zinc epitaxial relationships were summarized as:
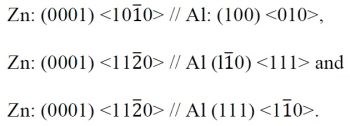
Alloy Substrates
The study reported above was only on pure aluminum; however, similar orientation relationships occur in the case of aluminum alloy substrates. An example of a typical zincated alloy 6061-T6 is shown in the bright-field and dark-field TEM micrographs in Fig. 3 and the corresponding selected-area diffraction pattern shown in Fig. 3c. Only aluminum reflections are present in the selected-area diffraction pattern. The zinc crystallites show up in bright contrast in the dark-field micrograph. The similarity of this diffraction pattern with the pure aluminum pattern of the same orientation11 implies that the same epitaxial relationships exist. The morphology of the deposits differs, as a comparison between Figs. 2 and 3 shows. The number of separate crystallites is far less on the alloy, even though their habit (six-fold symmetry, three-fold symmetry, etc.) remains the same. Similar studies on other alloys seem to confirm that the orientation relationship found on pure aluminum also holds for the alloys.
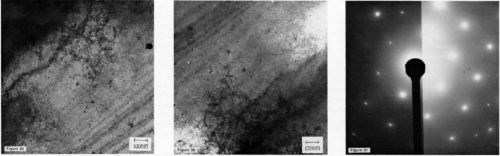
Figure 3 - TEM micrographs of zincated 6061-T6: (a) bright field, (b) dark Field and (c) selected area diffraction pattern from (b) (100 keV).
Electrochemical effects in the vicinity of inclusions and solute concentrations, which occur at grain boundaries, for example, have an important influence on deposit morphology. For instance, a specimen of 97% aluminum whose principal impurities were magnesium and iron was metallurgically polished, then zincated in Solution II and, as can be seen in Fig. 4a, was observed to have grown the zinc crystallites predominantly at the grain boundaries. On pure aluminum (99.999%), this behavior was not observed. The morphology of a tin deposit on the same alloy following a stannate treatment is shown in Fig. 4b. The tin seems to deposit along zones on either side of the grain boundary which presumably are electrochemically different from the material in the grain interior.
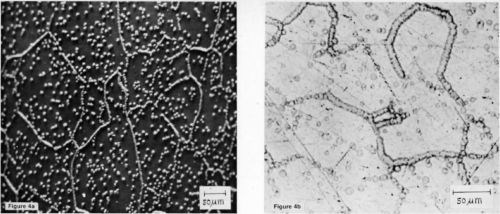
Figure 4 - (a) SEM micrograph of zinc deposits at grain boundaries in 97% aluminum containing both iron and magnesium (20 kev): (b) SEM micrograph of tin deposits al grain boundaries on the same alloy.
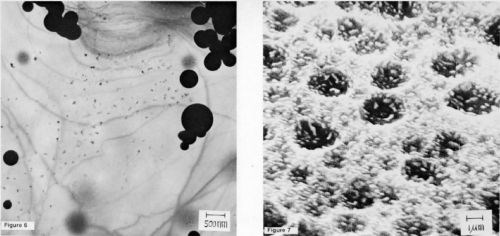
It would seem from the appearance of these micrographs that the stannate process responds somewhat differently than docs the zincate process. The procedure recommended by the supplier* is also different. Namely, following a similar cleaning step, the article to be coated is first immersed in the proprietary alkaline tin solution, where the aluminum oxide is thought to be stripped off; however, unlike the zincate process, the article is then transferred, without rinsing, to a bronze electrolyte, with electrical contact made prior to entering the solution. A TEM specimen subjected to this treatment with a 5-sec (3.2 A/dm2) bronze strike is shown in Fig. 5a. If bronze plating is continued, the crystallites will coalesce to form a continuous film.
The preferred orientation of the bronze is clearly apparent in the selected-area diffraction pattern of Fig 5b. However, if the specimen is rinsed following the tin immersion, a tin coating is repeatedly observed, as shown in the TEM micrograph of Fig. 6. In fact, adhesion measurements, to be reported in the future, indicate that on pure aluminum no deleterious effects are introduced by rinsing following the tin immersion; however, on alloys, the adhesion is significantly reduced. These results indicate that, at least on pure aluminum, a thin coating of tin exists underneath the bronze. Tin is clearly present on an etched specimen of pure aluminum (99.99%), as shown in the SEM micrograph of Fig. 7. Thus, it seems that tin is deposited by immersion on pure aluminum in much the same way as zinc is deposited.
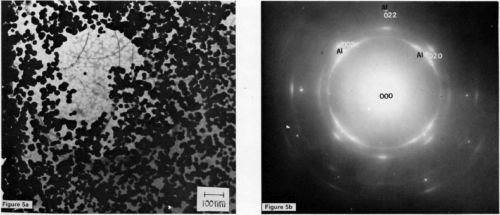
Figure 5 - (a) A TEM micrograph of 99.999% aluminum subjected to an immersion tin treatment followed by a 5-sec bronze strike (100 keV); (b) selected-area diffraction pattern from the above area.
Aluminum dissolution
Both the stannate and zincate processes require the dissolution of aluminum. This dissolution is not a random process, but rather, occurs on some crystallographic planes at a faster rate than on others. In order to further investigate the effects of crystallography on the immersion deposition process, a single-crystal sphere was fabricated in the manner described previously. The advantages of investigating a coated sphere become apparent when it is realized that each plane is tangent to the surface at one point or another.
|
|
|
|
Figure 6 - Dark-field micrograph of tin deposit on etched 99.99% aluminum (100 keV). |
Figure 7 - SEM micrograph of tin deposit on etched 99.99% aluminum (100 keV). |
The etch patterns caused by aluminum removal in either the zincate or the stannate solutions are exactly the same, and, as might be expected, correspond to that resulting from etching in a caustic solution, as has been confirmed by numerous experiments. Optical micrographs of a 25-mm sphere, which was zincated for 1 min, rinsed, then dipped in a 50% nitric acid solution (to improve the contrast of the etch pattern for the photograph), are shown in Fig. 8. The etching seems to be most severe in those high-index planes surrounding the (100) plane and extends along the (110) plane zones. The nitric acid dip does not change the etch pattern. The zinc coverage on the sphere was apparently too thin to allow Laue back-reflection patterns to be made, so that TEM had to be relied upon to obtain the orientation relationships.11
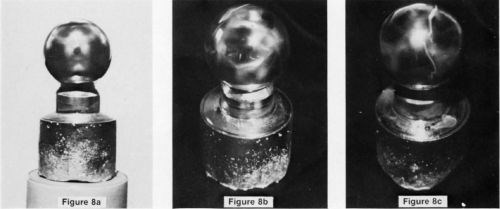
Figure 8 - Optical micrographs of a single crystal sphere subjected to a 1 -min zincate immersion followed by a dip In 50% nitric acid to strip off the zinc (a) the (100) plane, (b) the (110) plane and (c) the (111) plane.
Double zincate
The process known as the double zincate is commonly used and reported to result in improved adhesion.3 This process includes a zincate immersion, a rinse, a nitric acid dip to strip off the surface zinc, a rinse, followed by a final zinc immersion. Specimens subjected to various steps of the double zincate treatment were examined using a nuclear microanalysis technique, with 1.8 MeV He4 ions to induce nuclear reactions. Reaction products are scattered at specific energies and angles characteristic of the material on the surface. The sensitivity of this technique can be as high as 1012 atoms/cm2, the results being independent of the chemical or physical binding of the nuclei in the matrix. The results of these experiments are summarized in Table 2. The zincate solution used in these specimens contained ferric chloride and Rochelle salts; thus, it is not surprising that iron (from the ferric chloride) was deposited along with the zinc. In specimens subjected to a single zincate treatment, there was so much iron on the surface that the iron and zinc peaks overlapped and precluded a quantitative determination.
Table 2 - Helium backscatter data from 99.99% aluminum substrates processed in various ways.

After the zinc was stripped in a solution of 50% nitric acid for 30 sec and then thoroughly rinsed, both zinc and iron still were detected on the surface in roughly equal quantities of about two monolayers. Tin was found on the surface of specimens subjected to a proprietary stannate immersion treatment* in a quantity of about 15 monolayers.4 From these results, it can be concluded that iron plays an important role in the deposition process and, moreover, that a surface alloy on the aluminum exists that is comparatively insoluble in nitric acid. These conclusions are supported by transmission electron microscopy of specimens subjected to a double zincate treatment in Solution II. As is seen in Fig. 9, a number of rectangular regions exist which, in some cases, protrude into a preformed hole in the specimen, indicating a lack of solubility in the acid solution.
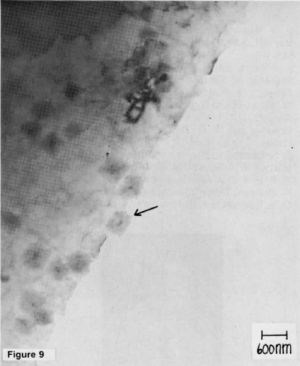
Figure 9 - A TEM micrograph of a specimen subjected to a nitric acid immersion.
Discussion and conclusions
It has been shown above that the substrate orientation plays an important role in the zincate process. Not only is the dissolution of the aluminum in the caustic zincate solution a sensitive function of the crystallographic orientation of the substrate but, also, the zinc itself, as is shown in a separate paper,11 forms epitaxial deposits at least on the principal planes of aluminum. In virtually every description of a process for plating on aluminum, a caustic cleaning step is specified. The highly deformed surface usually present on most materials subjected to mechanical polishing, machining, etc., interferes with epitaxial phenomena. In addition, this highly deformed layer would be expected to dissolve rapidly, compared to less highly deformed material, permitting a thick zinc deposit to form rapidly. By incorporating a caustic cleaning step prior to zincating, the necessary balance between the rate of oxide and aluminum dissolution with the rate of zinc deposition is maintained. Because the etching of the aluminum is a selective phenomenon, (the most active material is removed first), it would be expected that material which has been over-etched would exhibit a much slower rate of zinc deposition.
The effects of alloy constituents also play an important role in the zincating process. It was shown that even small traces of magnesium determine the sites at which large zinc crystallites grow. The epitaxial phenomena observed on pure aluminum were also observed on one alloy (6061-T6) and most probably also exist on others.
It was shown that small additions of ferric chloride have a major effect on the immersion reaction. Without ferric chloride and Rochelle salt, three atoms of aluminum dissolve for every two zinc atoms deposited. With ferric chloride present, nine atoms of aluminum must pass into solution before one zinc atom can be deposited. Moreover, it appears that an alloy, most probably a zinc-iron, is formed in deposits from zincate solutions containing ferric chloride. Some of this material was shown to remain on the surface following a dip in 50% nitric acid as is normal in the double zincate procedure.
This study on the morphology of zinc immersion deposits on aluminum has revealed the reasons behind a number of steps in the zincate process and also has shown the similarities between it and other immersion techniques for plating on aluminum.
Acknowledgments
The author thanks the Aluminum Association and the American Electroplaters' Society for their financial support of this program and is also grateful to Fielding Ogburn and Dr. Jerome Kruger for a critical review of the manuscript. Special thanks are due to Professor B. Agius of the Groupe de Physique des Solides de l'Ecole Normale Superieure for the nuclear reaction analysis.
References
1. S. Wernick and R. Pinner, The Surface Treatment and Finishing of Aluminum and Its Allovs, Vol. 2, Robert Draper Ltd., Teddington, U.K., 1971; p. 898.
2. H.E. Chandler and D.F. Baxter Jr., Metals Progress, p. 53 (January 1979).
3. F. Keller and W.G. Zelley, J. Electrochem. Soc., 97 (4), 143 (April 1950).
4. G. L. Bailey, J. Electrodepositors Tech. Soc., 27, 233 (1951).
5. R.V. Vanden Berg, Aluminum, Ed. bv K. R. Van Horn, Vol. III, ASTM publication, Metals Park. OH, 1967; p. 685.
6. W. Bullough and G.E. Gardam, J. Electrodepositors Tech. Soc., 22, 169 (1946-47).
7. D.S. Lashmore, Plating and Surface Finishing, 65 (4), 44-47 (1978).
8. ASTM B253-11, Standard Guide for Preparation of Aluminum Alloys for Electroplating, ASTM International, West Conshohocken, PA, 2017, www.astm.org
9. T.P. Dirkse, J. Electrochem. Soc., 101 (6), 328 (1954).
10. H. Benston, Trans. Electrochem. Soc., 88, 307 (1945).
11. D.S. Lashmore, J. Electrochem. Soc., 127 (3), 573 (1980).
About the author (at time of publication)

Dr. David S. Lashmore received his Ph.D. in Materials Science from the University of Virginia in June 1977 and is currently Technical Director of AES Research Project 41, "Plating on Aluminum." He obtained a B.S. degree in engineering science and mechanics from the University of Florida in 1968, and a M.S. degree in physics from Michigan Technological University in 1970. He was the recipient of a National Science Foundation Undergraduate Research Grant in 1968 and was awarded the Thornton Fellowship for 1975-76 by the University of Virginia. He has worked as a physics instructor and in other capacities and has a number of publications. He is a member of Sigma XI.
* M&T Chemicals, Inc., Rahway, NJ.
Related Content
Innovation in Plating on Plastic
Plating on advanced plastics solution offers improved adhesion, temperature resistance and cost savings.
Read MoreProducts Finishing Reveals 2023 Qualifying Top Shops
Each year PF conducts its Top Shops Benchmarking Survey, offering shops a tool to better understand their overall performance in the industry. The program also recognizes shops that meet a set of criteria to qualify as Top Shops.
Read MoreTrivalent Chrome Overview
As the finishing industry begins to move away from the use of hexavalent chromium to trivalent chromium, what factors should finishers consider as they make new investments? Mark Schario, chief technology officer for Columbia Chemical offers a helpful overview of this complicated topic.
Read MoreNanotechnology Start-up Develops Gold Plating Replacement
Ag-Nano System LLC introduces a new method of electroplating based on golden silver nanoparticles aimed at replacing gold plating used in electrical circuits.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More