Avoiding White Spots Caused by Galvanic Corrosion on EN AW 2024
Four approaches to troubleshoot white spots that appear because of galvanic corrosion.

Q. How do you avoid undesirable white spots caused by galvanic corrosion during the anodizing process on aluminum EN AW 2024 anodized black surface?
White spots are a challenging phenomenon in anodizing as it can be tough to understand the cause. This article presents four approaches to troubleshoot white spots that appear because of galvanic corrosion.
1) Aluminum alloys
The aluminum alloy EN AW 2024-T3511 is one of the alloys that often causes problems with white spots. The reason for this is the content of the alloying elements copper and magnesium, as well as the amount of iron. These alloying elements form different phases in the microstructure, which can cause galvanic corrosion. It is well established that the EN AW 2024 aluminum-copper alloy creates inhomogeneous oxide layers in the areas of the intermetallic phase, CuAl2 and other copper containing phases. This alloy is both more sensitive to galvanic corrosion, but also more prone to pitting corrosion.
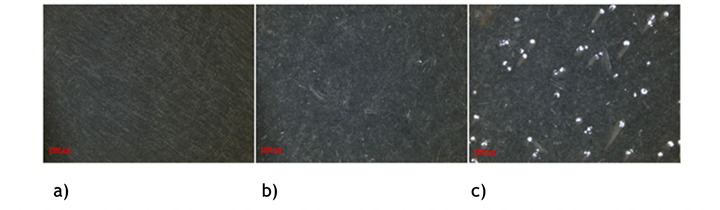
Figure 2: Stereo microscope images of a) anodized, black EN AW 2024, b) anodized, black with small white spots c) anodized, black and with a severe amount of spots.
As seen in image c), a spot arising from galvanic corrosion is often tailed but with no specific direction. This indicates that the spots are not dependent on agitation of the solution or other directional issues.
2) Pre-treatment process
If the microstructure is inhomogeneous and consists of copper-containing intermetallic phases, it is crucial to make sure that no chloride is used in any of the chemical pretreatment steps. This also means checking the rinse water tanks used in the pre-treatment step as they are often filled with city water. A change in the incoming water can be detrimental for production. Chlorides are a guarantee for white spots on EN AW 2024 alloys.
Moreover, shortening down process time or even eliminating the pretreatment steps with a very high pH (alkaline) or very low pH (acidic), can diminish the appearance of these corroded spots.
3) Anodizing process
When anodizing EN AW 2024, an inhomogeneous anodic layer is formed with varying layer thickness and cracks (Figure 3).

Fig. 3: SEM image of a black anodized surface seen from the top.
These cracks create areas with weak spots in the oxide layer, leaving the surface less corrosion-resistant but also open for a small galvanic current to run. This happens because weak spots are more anodic than the rest of the coherent oxide layer, creating small galvanic cells and leading to a corrosion attack found on the black surface as white spots. Mostly, this is first observed after the coloring or sealing process.
Like in the pre-treatment step, chlorides in the anodizing tank caused by sulfuric acid or additives can be the reason for white spots. The same is true for chlorides in the dye or sealing additives.
The rinse water should also again be checked for chloride as the surface is even more prone to galvanic attack in the anodizing step. The reason for this is the formed oxide layer is non-conductive so if there is a current, there are only small spots for it to attack. This can lead to severe galvanic corrosion attack as seen in Figure 4.

Fig. 4: SEM image of a black anodized surface seen from the top with a lot of galvanic attacks.
Creating a homogeneous and coherent oxide layer on these alloys can be done by using various anodizing parameters such as ramping, pulsing and change in concentration of the sulfuric acid.
4) Titanium racks
Using titanium racks can increase the possibility for galvanic corrosion because the titanium itself can contain a small current from the anodizing process. This small current is created because of the ability of titanium to act as a capacitor out of the anodizing tank. Magnesium plates can be used to solve this issue. The magnesium surface acts as a sacrificial anode, designed to corrode before the aluminum does. The contact is important between the titanium rack and magnesium plate, and it is necessary to secure that no stray current can run. This can be done by shutting down the electrical contact to the coloring tank under the coloring process and cleaning the surface of magnesium.
About the Author

Anne Deacon Juhl
Anne is the owner of AluConsult and
Anodizing School.
Visit anodizingschool.com
Related Content
Anodizing for Bonding Applications in Aerospace
Anodizing for pre-prep bonding bridges the gap between metallic and composite worlds, as it provides a superior surface in many applications on aluminum components for bonding to these composites.
Read MoreChicago-Based Anodizer Doubles Capacity, Enhancing Technology
Chicago Anodizing Company recently completed a major renovation, increasing its capacity for hardcoat anodizing and Type II anodizing.
Read MoreFinisher’s ‘Top Shop’ Status Attracts Business
This competitive California finisher made it a goal to become a PF Top Shop. After earning the recognition, the company experienced an immediate increase in business and a challenge to obtain certifications.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More











.jpg;maxWidth=300;quality=90)









