Color of Brass Deposits
What should be the ratio of copper to zinc metal in a cyanide-brass plating bath if I am trying to obtain a gold-colored finish?
Q. I am trying to obtain a gold-colored finish in a cyanide-brass plating bath. What should be the ratio of copper to zinc metal in this bath? Any other information that you can give me also would be helpful.— W.J.
A. The table below gives you the color of brass deposits depending on the amount of copper in them:
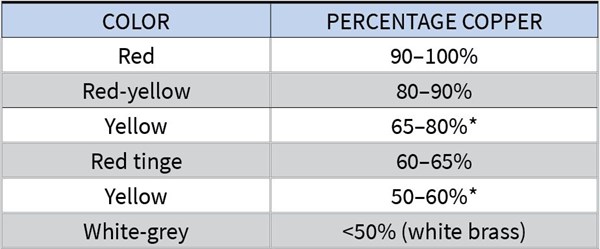
The two ranges marked with an asterisk (*) will give you a gold-colored deposit.
A typical brass plating bath has the following components and operating parameters:
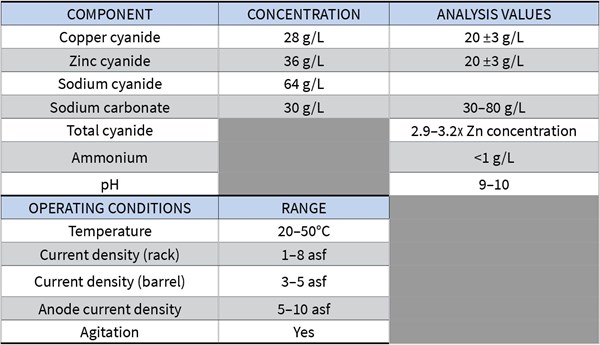
You will have to do some experimentation using a Hull cell to find the formulation that matches the gold color you are looking for.
Related Content
-
How to Maximize Nickel Plating Performance
The advantages of boric acid-free nickel plating include allowing manufacturers who utilize nickel plating to keep up the ever-changing regulatory policies and support sustainability efforts.
-
Liquid Chrome Vs. Chromic Acid Flake
Contemplating how to continue offering chromic acid services in an increasingly stringent regulatory world? Liquid chrome products may be the solution you’re looking for.
-
A Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.
















