Zirconium-based conversion coatings have been around for more than a decade and are used globally in a variety of applications. They also are fast becoming the preferred choice for pretreatment over traditional iron phosphates, and in some cases, zinc phosphates. There are several differences between zirconium and phosphate coatings, resulting in both advantages and disadvantages.
Advantages
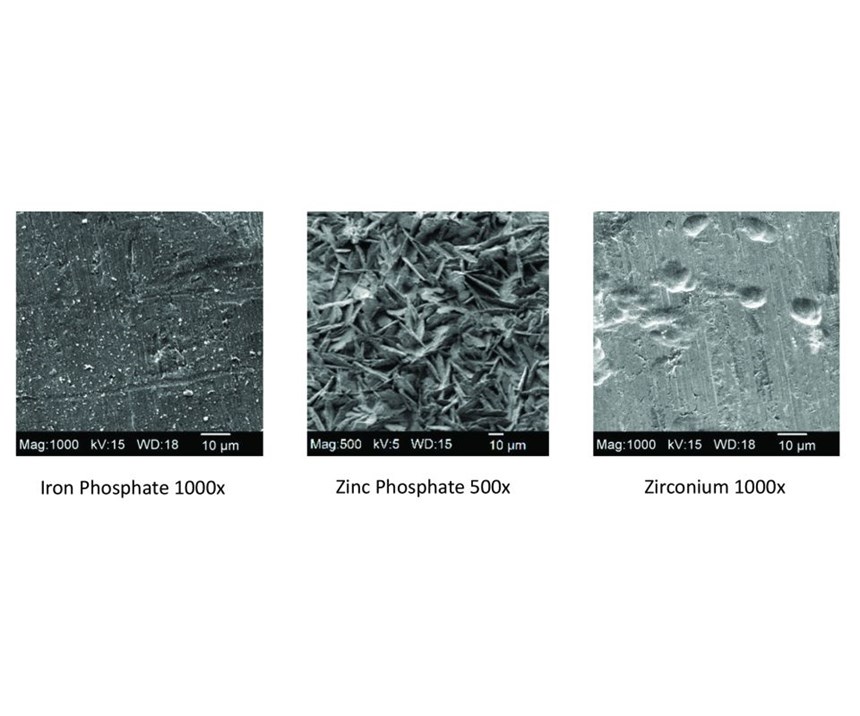
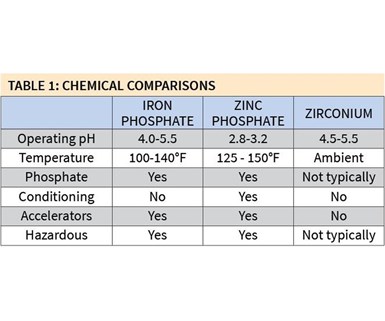
Table 1 shows the chemical differences among iron phosphate, zinc phosphate and zirconium coatings. The operating pH of iron phosphates varies between 4.0 and 5.5, which is very similar to that of zirconium coatings, while the pH of zinc phosphate coatings typically is much lower. However, the first major advantage of zirconium coatings is their ability to operate at ambient temperatures. This can provide significant energy savings, depending on the temperature at which a shop currently operates its iron phosphate line.
A second advantage is that most zirconium formulations on the market do not contain phosphates, which many local municipalities currently do not allow to be discharged into public systems at all or only in very limited amounts. By converting to a phosphate-free technology, shops avoid this problem.
Zinc phosphate also is the only chemistry among the three that requires a conditioning step to initiate crystal formation. Without this step, the zinc phosphate coating would be very sparse and the crystal structure very large. Both zinc and iron phosphates also require accelerators in order to achieve a uniform and quality coating. Most zirconium coatings do not require accelerators.
A third advantage of some of zirconium coatings is that, unlike their phosphate-based counterparts, they are nonhazardous to the health and safety of operators.
Table 2 shows some of the major physical differences among the three types of coatings. Iron phosphates are mostly manufactured in liquid form, although there are still a few powder formulations available on the market today. Zinc phosphates and zirconium coatings are produced in liquid form.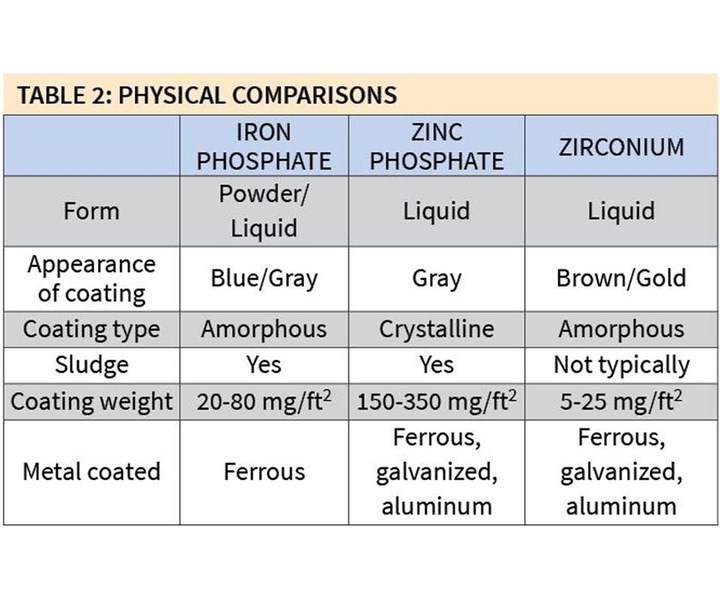
The fourth advantage of zirconium coatings over phosphate coatings is that the process produces very little if any sludge, because the reaction of the zirconium chemistry is typically about 99-percent efficient, leaving little in the form of non-deposited coating or sludge. Conversely, both iron phosphate and zinc phosphate coating processes are very inefficient and produce significant amounts of sludge.
Coating weights for zirconium coatings also are typically very low: 5-25 mg/ft2. This is why they are often referred to as thin-film coatings, and nanocoatings or nanotechnologies. The weights of iron phosphate coatings are higher and those of zinc phosphate coatings are much higher.
Yet another advantage to zirconium coatings is that they can be effectively used on all three major substrates of metal: steel, galvanized and aluminum. Iron phosphate formulations can only produce quality coatings on ferrous substrates, requiring the element of iron. A typical drum of iron phosphate does not itself contain any iron, so it is dependent on the metal being processed to provide the source of iron. Therefore, when the process is used on galvanized or aluminum metals, very little, if any, iron phosphate is actually deposited.
In terms of process equipment, 316/314 stainless steel is preferred, although not required, for iron phosphate and zirconium coatings, but it is a requirement for zinc phosphate coatings (see Table 3). Pumps for all coatings should be stainless steel.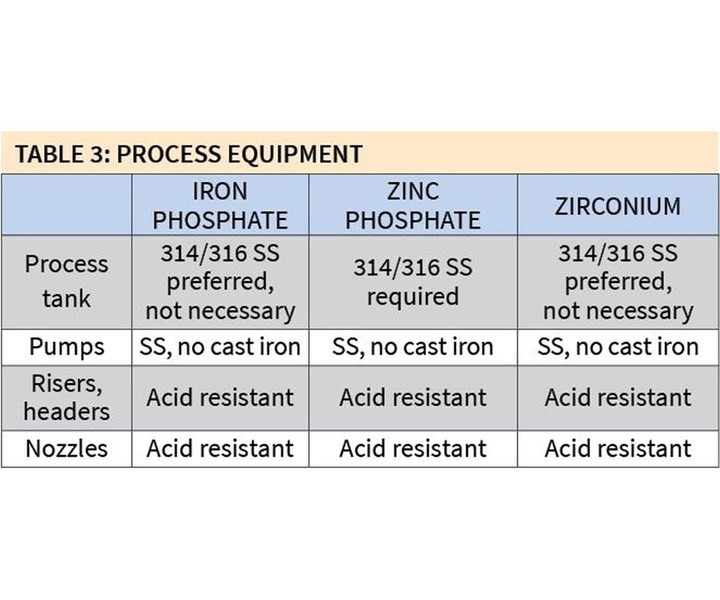
A typical five-stage iron phosphate process (see Figure 1) will start with an alkaline cleaner, followed by a fresh and overflowing city-water rinse, then the heated iron phosphate coating stage. This coating stage will be followed by an overflowing rinse with city water, or deionized or reverse osmosis water. The fifth and final stage of the process will be sealing.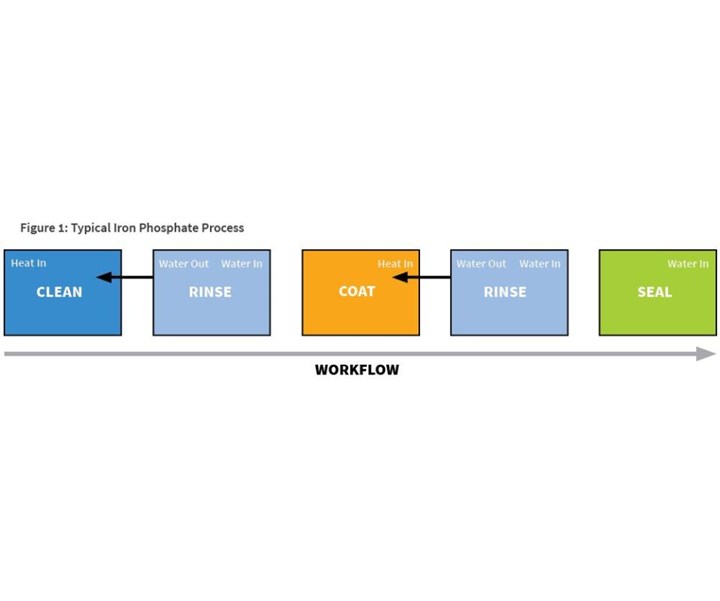
This five-stage process requires a significant amount of energy (both the cleaning and coating stages are heated), and a significant amount of water (two overflowing rinses and two evaporating stages). In addition, there is the potential for carryover or cross-contamination of chemicals, thanks to the single rinses between the chemical stages.
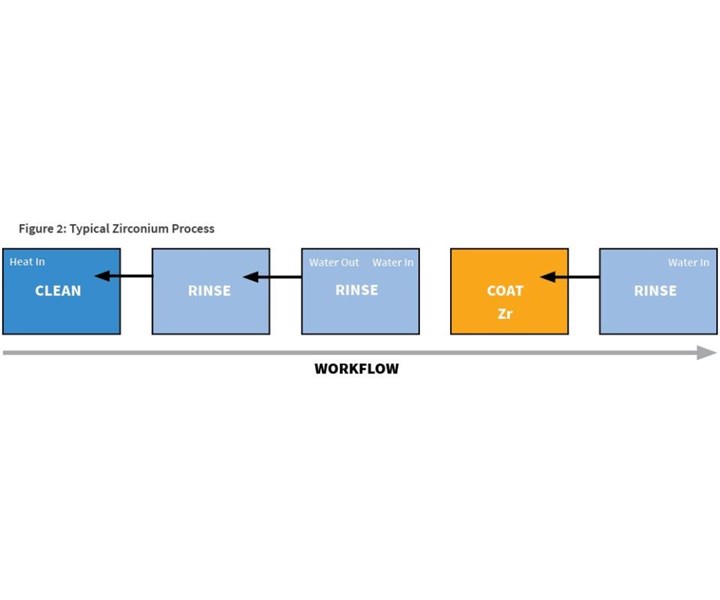
The typical zirconium coating process also is a five-stage process (see Figure 2), but it is much different from the iron phosphate process. First, there are only two chemical stages: cleaning and coating; sealing is not required. Also, there are two rinses between the cleaning and coating stages, and only the cleaning stage is heated. All rinses are counter-flowed, resulting in much less water usage and less chemical waste.
As mentioned earlier, zirconium coating processes produce very little if any sludge, allowing for long bath life. The typical zinc phosphate bath should last about one to three months, an iron phosphate bath three to six months, and a zirconium bath six to 12 months or longer. Placing small cartridge filters, typically of 20 to 30 microns in size, in these baths also can significantly extend bath life.
Disadvantages
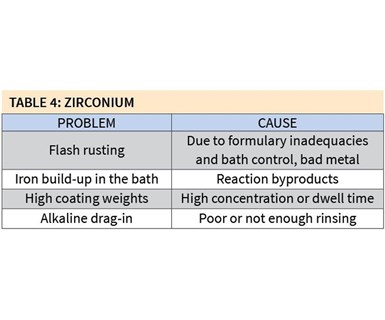
Zirconium coating does not necessarily come without some major problems, however (see Table 4).
Flash rusting can occur as a result of formulary inadequacies and poor bath control, or the quality of the metal being coated. One of the ways to remedy this problem is to add a small amount of alkaline neutralizer to either the stage before or the stage after the zirconium bath. The bath’s pH also can be raised as high as 6.0 to help reduce the amount of flash rusting.
Iron buildup in the bath is a common reaction and byproduct of zirconium coating. In addition to extending the life of the bath, as mentioned above, adding a small cartridge or bag filter, typically 20 to 30 microns in size, can help significantly reduce iron buildup without increasing chemical consumption.
High coating weights greater than 30 mg/ft2 can result if the bath’s concentration is too high or the dwell time in the coating stage is too long. A dwell time of 30–45 seconds should be sufficient, and temperatures between 60°F and 100°F are optimum for coating deposit.]
Alkaline drag-in may occur if there is not sufficient rinsing between the alkaline cleaner stage and the zirconium coating stage. Unlike iron phosphate coatings, zirconium coatings are very sensitive to alkaline contamination, so the process should include at least two rinses between the cleaner and coating. If an established process has only one rinse, a pH neutralizer should be added to constantly reduce the alkalinity of that rinse stage.
Converting Iron Phosphate to Zirconium
There are several options for those with less than a five-stage system. Conversion coatings are available that allow cleaning and application of a zirconium coating in a single stage on all three key metals types.
Converting a five-stage iron phosphate process to a zirconium coating process is relatively simple. The zirconium chemistry will replace the rinse stage of the iron phosphate process, also making it less expensive to operate and convert; remember, zirconium coatings do not require heat. Also, the existing, larger iron phosphate stage will be converted into a rinse stage, and cleaning of this stage will not be as critical because it will not contain chemistry. Finally, since the sealing stage also is being replaced with a freshwater rinse, that stage probably already is fairly clean as well.
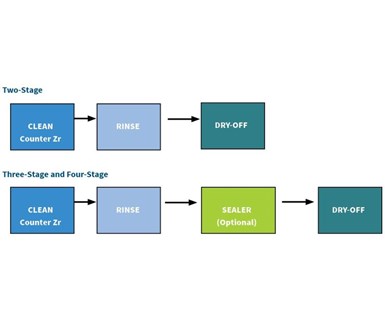
So, the basic layout for the converted five-stage line will be alkaline clean, rinse, rinse, zirconium coating, rinse. But what if your current line only has two, three or four stages? Cleaner/coater conversion coatings currently are available that allow both cleaning and application of a zirconium coating in a single stage on all three key metals types. Some work better with a final seal and some without (see two-, three- and four-stage examples).
Potential Cost Savings
The potential costs savings from converting from a zinc phosphate pretreatment line to a zirconium line can be substantial. In one case study, converting an 11-stage phosphate line to a zirconium line produced total annual savings of $350,000. This included savings of $150,000 in energy, $80,000 in waste disposal, $20,000 in lower maintenance costs and $100,000 in lower chemical costs. This immersion system included a 10,000-gallon tank, and operating temperature was 140°F.
A second case study of the conversion of a five-stage iron phosphate line produced approximate annual savings of $73,000. This number included savings of $36,000 in energy costs, $15,000 in waste disposal, $10,000 in lower maintenance costs and $12,000 in lower chemical costs. This was a spray system with a 2,000-gallon tank and operating temperature of 120°F, and the tanks were dumped every three months.
Environmental Differences
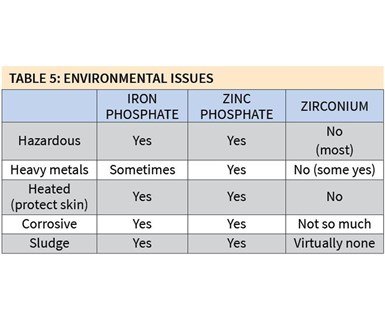
Some of the environmental differences among iron phosphate, zinc phosphate and zirconium coatings are significant (see Table 5). As mentioned earlier, many zirconium coatings are non-hazardous, while both iron and zinc phosphate coatings are. Also, very few zirconium coatings contain heavy metals, while all zinc phosphate and some iron phosphate coatings do. Zirconium coatings also are much less corrosive and produce very little, if any, sludge.
To summarize, there are many reasons that zirconium coatings can be considered an excellent replacement for iron and zinc phosphates:
- They can coat any metal.
- They are environmentally friendly.
- They operate at room temperature.
- They produce virtually no sludge.
- They are phosphate-free.
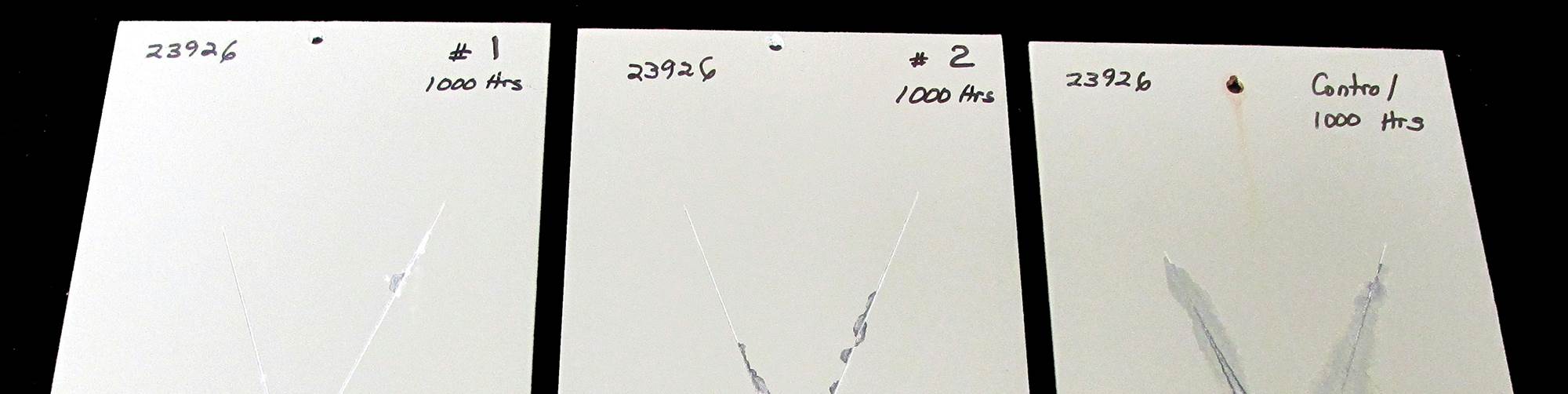
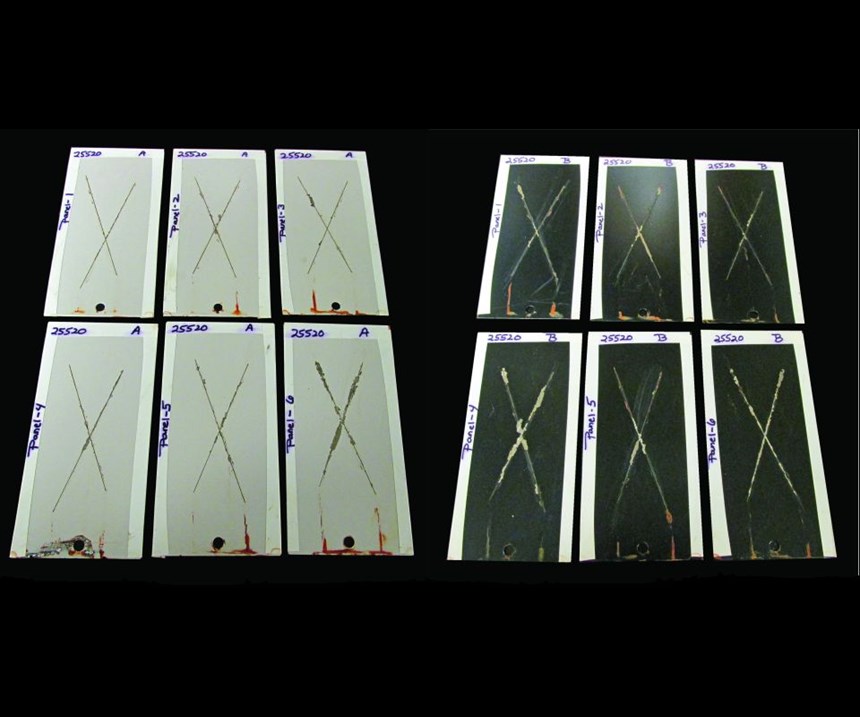
- They outperform iron phosphates and are as good as zinc phosphates in salt spray testing.
- Their operational costs are lower than those of both zinc and iron phosphates.
- They can be easily implemented in existing conventional processes.
-
About the Author
Sergio Mancini is director of sales for Bulk Chemicals. He has a degree in chemistry from Rutgers University and more than 40 years’ experience in the metal finishing industry. Visit bulkchemicals.us.
Related Content
Zinc Phosphate: Questions and Answers
Our experts share specific questions about zinc phosphate and pretreatment
Read MorePrevent Plating Problems with Critical Inspections
Tanks and their contents should be regularly inspected visually and analytically. When a quality issue arises, it is important to quickly pinpoint where the main problem is by checking which parameter is out of line.
Read MoreA Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.
Read MoreRead Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More