Desmutting is the act of removing excess alloyed metals from the surface of aluminum after etching, and it can be done using any mineral inorganic acid, such as hydrochloric, sulfuric and nitric acids. Deoxidizing is different from desmutting in that it removes aluminum oxide off the aluminum substrate via redox (or oxidation-reduction) reactions, something normal mineral acids cannot achieve. You can always desmut aluminum, but you may not always be able to deoxidize aluminum prior to processing.
Corrosion occurs when bare aluminum is exposed to oxygen and moisture. Extended time in a deoxidizer will remove and inhibit further corrosion of the metal.
Chemical processes common to preparing the metal for such surface finishing as anodizing, plating or painting put aluminum at risk for corrosion. Interestingly, aluminum inherently protects itself from this corrosion in stable conditions (pH of around 4.5-8.5) by readily oxidizing—creating a very thin layer of aluminum oxide when it comes in contact with the surrounding oxygen. This layer of oxide is what we seek in anodizing, but it is not of a sufficient thickness to serve our applications. Thus, we need to remove it with a solution called a deoxidizer, which intentionally exceeds this pH range.
Of course, deoxidizing of aluminum can only be successful if there is aluminum oxide present on the part or there is a chemical species willing to donate or accept electrons in the solution. The key constituents in the chemical solution are ferric sulfate and nitric acid. Since ferric is in the +3 state, it can be the oxidizing agent and reduce the aluminum oxide on the surface. The nitric acid is very important because of its inherent oxidizing abilities, which help speed up the reaction and decrease the amount of time the part spends in the deoxidizer. Once the thin layer of aluminum oxide is gone, you are left with bare aluminum that has been activated, meaning it is looking to donate electrons to maintain equilibrium. This is why deoxidizing should be done right before anodizing or plating.
Types of Deoxidizers
The most commonly used acid solutions for deoxidizing are nitric-, sulfuric- or chromic-acid based. Nitric and sulfuric solutions are usually interchangeable or paired, so I will only discuss nitric-based deoxidizers, which are more common, and chromic-acid based ones.
This is a typical tank setup for deoxidizing aluminum parts. Notice that the tank is made of a plastic material in order to prevent chemical reactions from occurring with its walls.
Nitric acid is the more common choice for non-etching deoxidizing because of its ability to slowly attack aluminum and because of its autocatalytic abilities as an oxidizer. Nitric-acid deoxidizers are usually light-duty solutions and are principally employed as desmutting agents. They will produce satin-type finishes without removing the finished-metal shine. Nitric-acid deoxidizers also are useful for salvaging parts, as nitric acid is known to open the pore structure of anodic films, allowing for easier stripping of anodize.
This is an effective deoxidizer/desmutting solution that can be used at room temperature without the need for fume exhaust. Immersion time is 1-5 minutes, and triple rinsing is the most effective way of removing the solution. Nitric-acid-based deoxidizers are typically considered more environmentally friendly, too, since there is no chromium involved. Tanks can be made of 316 stainless steel or polypropylene, or have some sort of polyvinylidene fluoride (PVFD) liner.
Chromic-acid-based non-etching deoxidizers are the gentlest of all deoxidizers. They will passivate bare aluminum and tend to confer a passivating action on any other solution to which they are added. These types of deoxidizers are popular for removal of heat-treat films and to prepare aluminum alloys for zinc immersion plating, chromic-acid anodizing, painting and other chemical treatments. They help eliminate surface irregularities caused by oxide inclusions or embedded buffing particles, and generally do not remove nearly as much metal as caustic soda while leaving the surface of the metal clean and semi-bright. Chromium-based deoxidizers work best in tanks made from 18-8 stabilized, stainless steel-clad material or lined with lead, PVFD or high-density polypropylene.
Casting is a common manufacturing process, but it usually produces a rough surface that is not ideal for surface finishing. Smoothing the surface is best achieved with a heavy-duty deoxidizer.
Hydrofluoric-acid-based deoxidizers are aggressive and can be used on castings, or shot-peened or blasted parts, providing a light etch and a matte finish. These deoxidizers are very effective on casting alloys because of the fluoride’s ability to dissolve silica, a primarily chemically inert element that is used to make glass and in aluminum castings.
All of these acid types are dangerous and proper operator safety precautions should be taken.
Deoxidizer Applications
Deoxidizers can be used in a variety of ways to help prepare aluminum surfaces for subsequent processing and to help salvage parts that have failed final inspection. As mentioned, they are appropriate for
- Cleaning with little or no etching.
- Desmutting or as a neutralizing rinse after alkaline etching.
- Removal of oxide, mill scale, corrosion products, heat-treatment scale or welding fluxes.
- Etching to increase surface area.
- Heavy-duty deoxidizing for castings.
- Light-duty deoxidizing.
In the aerospace industry, for instance, the parts are complex and have tight tolerances for which etching off of material is not acceptable. Materials of choice are 2000 and 7000 series alloys of copper, zinc, magnesium and other metals. These intermetallic inclusions, especially in 7000 series metals, which yield superior strength and corrosion resistance, will react with the atmosphere almost readily. Most of the time, these parts do not present oiled or protected prior to finishing, so they often show oxide film buildup or the beginning of pit formation. A light-duty deoxidizing will remove any oxidation films or localized pitting before anodizing or plating.
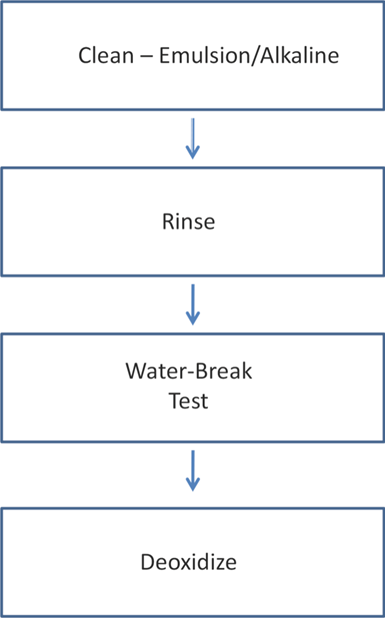
In order to deoxidize effectively, the metal surface must be clean to allow reaction with the redox chemicals. Cleaning and water-break testing are a must before deoxidizing.
Deoxidizers can also be used to fix welding fluxes and heat-treat scale on parts that were previously welded and underwent non-destructive testing. The welding marks can contain cavities and crevices that will hold electrolytic solution and could damage the part in post processing. Using a heavy-duty deoxidizer such as one with nitric and hydrofluoric acid can help reduce the weld cavities and make them smoother. Heat-treatment scale can prevent steels from becoming passive, and steels that are not passive will eventually rust, resulting in field failure. Hydrochloric acid can be used to remove such heat-treat scale.
A heavy-duty deoxidizer is the only type that can clean up the surface appearance of parts that are shot-peened, blasted or casted, and fluoride is the key to this deoxidizing method. A normal caustic etch could accomplish this, but it is much more difficult to control the metal removal. Casted alloys require a completely different process when it comes to surface preparation. Since these alloys are high in silica and other uncommon metals, the common practice of clean, etch, deoxidize cannot be used. Etching castings with alkaline solutions will cause severe cosmetic problems and paint adhesion failures. The high density of metallic inclusions on the surface of the part will cause voids in the anodize substrate, and this ultimately will cause the paint failure.
A bonus of using fluoride in deoxidizers is that it provides a frosty, matte finish that is cosmetically appealing and also gives a good finish for dye operations. Most decorative finishes will use deoxidizers with fluorides.
Maintaining the Deoxidizer
Obviously, facility controls are very important when you are running a plating line. Consistency is key with special processing, and the more controls you have, the more consistency. Any control with a measurable value should be calibrated, which will further help with consistency.
It also is important to make sure that the tank materials are acid-resistant, including the tank welds. Any tank that is heated also should have some sort of temperature indicator readily visible to the operator. This will help the operator gauge immersion times and will also prevent over-etching. A rule of thumb to remember is the reaction rate doubles for roughly every increase or decrease of 10°F. Say you remove 0.001 mil every 30 minutes in your deoxidizer at 85°F; if you raised the temperature to 95°F, your new etch rate would be around 0.002 mil every 30 minutes.
Another important must-have for a plating line is solution agitation. This will help keep both temperatures and concentrations uniform.
Manufacturing controls also can be implemented to help operators limit contact with pretreated metal parts. The sodium chloride found in body oils and sweat will eventually pit the aluminum if contact is made for enough time or if it is frequent enough. Operators should handle the parts wearing gloves that are clean, dry, and lint- and powder-free. A key to keeping contamination at a minimum is always having a clean supply of gloves at the operator’s station for frequent changes.
Quality control is just as important as facility and manufacturing control to keep the solution, equipment and operators from changing behaviors and delivering varied results. Solution control is a must, and chemical solutions should be analyzed weekly to ensure proper maintenance of composition, stability and etch rates.
Deoxidizing is an important first step in preparing aluminum for surface finishing. With the proper information and controls in place, redox chemicals will be able to react with the clean metal surface and make it ready for successful further processing.
Peter Totaro is a chemical engineer (CAF) and the quality manager for AeroTech Processing Solutions. Contact him at ptotaro@mastermetal.com. This article is based on a paper he presented at the 2017 Anodizing Conference at the Aluminum Anodizing and Extrusion Summit.
Related Content
An Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreConveyors and Paint Systems
Choosing the right conveyor system, coating technology, and ancillary equipment.
Read MoreHow to Choose Between Sulfate and Chloride-Based Trivalent Chromium
There are several factors to consider when choosing between sulfate and chloride-based baths for trivalent chromium plating. Mark Schario of Columbia Chemical discusses the differences and what platers should keep in mind when evaluating options.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More















.jpg;maxWidth=300;quality=90)








