Electro-codeposition of MCrAlY Coatings for Advanced Gas Turbine Applications - 9th Quarterly Report
The NASF Research Board has funded a research grant at the Tennessee Technological University, under the direction of Professor Ying Zhang. The objective is to study and optimize the MCrAlY electro-codeposition process to improve the coating oxidation/corrosion performance. In this reporting period, the study studied a sulfur-free fluoborate plating solution. Although the fluoborate solution is sulfur-free and offers high plating rates, the CrAlY powder was attacked by the fluoborate anion. The fluoborate-based plating bath may be suitable for codeposition of more inert particles but not for active CrAlY-based powders.
by
Prof. Ying Zhang* & B.L. Bates
Department of Mechanical Engineering
Tennessee Technological University
Cookeville, Tennessee, USA
Editor’s Note: This NASF-AESF Foundation research project report covers the ninth quarter of project work (January-March 2020) on an AESF Foundation Research project at the Tennessee Technological University, Cookeville, Tennessee. A printable PDF version of this report is available by clicking HERE.
Summary
In the first quarter of Year 3, the study focused on the exploration of a sulfur-free fluoborate plating solution for electro-codeposition of Ni-CrAlY composite coatings. Thick nickel coatings of 20-80 μm thickness were able to be plated in a relatively short time with the fluoborate bath and the coatings were uniform and adherent. However, when CrAlY powder was added, the fluoborate solution violently reacted with the powder and a uniform suspension could not be obtained. The resulting dark coating was powdery and loosely attached to the substrate. The coating surface exhibited poor electrical conductivity due to the presence of impurity elements such as F and O in the coating, as revealed by energy-dispersive spectroscopy (EDS) analysis. Although the fluoborate solution is sulfur-free and offers high plating rates, the CrAlY powder used in the codeposition process is readily attacked by the aggressive fluoborate anion. Therefore, the fluoborate-based plating bath may be suitable for codeposition of more inert particles but not for relatively active metal particles such as the CrAlY-based powders.
Technical report
I. Introduction
To improve high-temperature oxidation and corrosion resistance of critical superalloy components in gas turbine engines, metallic coatings such as diffusion aluminides or MCrAlY overlays (where M = Ni, Co or Ni+Co) have been employed, which form a protective oxide scale during service.1 The state-of-the-art techniques for depositing MCrAlY coatings include electron beam-physical vapor deposition (EB-PVD) and thermal spray processes.1 Despite the flexibility they permit, these techniques remain line-of-sight which can be a real drawback for depositing coatings on complex-shaped components. Further, high costs are involved with of the EB-PVD process.2 Several alternative methods of making MCrAlY coatings have been reported in the literature, among which electro-codeposition appears to be a more promising coating process.
Electrolytic codeposition (also called “composite electroplating”) is a process in which fine powders dispersed in an electroplating solution are codeposited with the metal onto the cathode (specimen) to form a multiphase composite coating.3,4 The process for fabrication of MCrAlY coatings involves two steps. In the first step, pre-alloyed particles containing elements such as chromium, aluminum and yttrium are codeposited with the metal matrix of nickel, cobalt or (Ni,Co) to form a (Ni,Co)-CrAlY composite coating. In the second step, a diffusion heat treatment is applied to convert the composite coating to the desired MCrAlY coating microstructure with multiple phases of β-NiAl, γ-Ni, etc.5
Compared to conventional electroplating, electro-codeposition is a more complicated process because of the particle involvement in metal deposition. It is generally believed that five consecutive steps are engaged:3,4 (i) formation of charged particles due to ions and surfactants adsorbed on particle surface, (ii) physical transport of particles through a convection layer, (iii) diffusion through a hydrodynamic boundary layer, (iv) migration through an electrical double layer and (v) adsorption at the cathode where the particles are entrapped within the metal deposit. The quality of the electro-codeposited coatings depends upon many interrelated parameters, including the type of electrolyte, current density, pH, concentration of particles in the plating solution (particle loading), particle characteristics (composition, surface charge, shape, size), hydrodynamics inside the electroplating cell, cathode (specimen) position and post-deposition heat treatment, if necessary.3-6
There are several factors that can significantly affect the oxidation and corrosion performance of the electrodeposited MCrAlY coatings, including: (i) the volume percentage of the CrAlY powder in the as-deposited composite coating, (ii) the CrAlY particle size/distribution and (iii) the sulfur level introduced into the coating from the electroplating solution. This three-year project aims to optimize the electro-codeposition process for improved oxidation/corrosion performance of the MCrAlY coatings. The three main tasks are as follows:
- Task 1 (Year 1): Effects of current density and particle loading on CrAlY particle incorporation.
- Task 2 (Year 2): Effect of CrAlY particle size on CrAlY particle incorporation.
- Task 3 (Year 3): Effect of electroplating solution on the coating sulfur level.
II. Background
A typical MCrAlY coating consists of 8–12% Al, 18–22% Cr, and up to 0.5% Y (in wt%). Other more complicated compositions of MCrAlYs contain additional elements such as hafnium, silicon and tantalum.7,8 The concentrations of some minor elements (e.g., sulfur, yttrium and hafnium) play an important role in affecting the growth and adhesion of the oxide scale. The detrimental effect of sulfur on oxide scale adherence of MCrAlY alloys has been well documented.9 Small amounts of sulfur can segregate to the alumina-metal interface and weaken the interface.10 An earlier study by Bornstein, et al.11 clearly showed the effect of concentrations of sulfur and yttrium on the cyclic oxidation resistance of NiCrAlY alloys at 1100°C. For NiCrAlY alloys containing 40 ppm S, an addition of 890 ppm Y could effectively mitigate the sulfur effect and an adherent oxide scale was maintained. However, for NiCrAlYs containing 300 ppm S, 4000 ppm of Y was not able to counteract the sulfur effect.
The electrolytes used to deposit the nickel or cobalt metal matrix for forming the MCrAlY coating are typically sulfate- or sulfamate-based solutions.12,13 Approximately 0.006-0.013 wt% (60-130 ppm) of sulfur has been reported in electroplated nickel coatings using these solutions.14,15 The Watts bath (sulfate-based) is one of the most commonly used electrolytes, which was also employed in our previous electro-codeposition process for fabricating NiCoCrAlY coatings. Two sulfur-free baths, i.e., the fluoborate solution and the all-chloride solution, will be investigated. In this report, the electrodeposition results with the fluoborate-based solution will be summarized. The fluoborate solution is often used for heavy nickel plating and electroforming. The advantage is that it is possible to work with high nickel concentrations and therefore with high current densities.16 As a result, high deposition rates (e.g., ~100 μm/hr) have been reported.17
II. Experimental Procedure
3.1. Electroplating and electro-codeposition
Both electroplating and electro-codeposition experiments were carried out in the fluoborate bath in a glass beaker. The solution consisted of 220 g/L of nickel fluoborite, Ni(BF4)2 and 30 g/L of boric acid, H3BO3, mixed with deionized water. The substrate material used was Ni-200 (>99.0 Ni, with 0.40 Fe-0.35 Mn-0.25 Cu-0.35 Si-0.15 C-0.01 S max., in wt%). A baseline nickel coating was first plated without CrAlY powders. Ball-milled CrAlY powder (30 g/L) was then added to the solution to deposit the Ni-CrAlY composite coatings. The electro-codeposition setup is illustrated in Fig. 1.
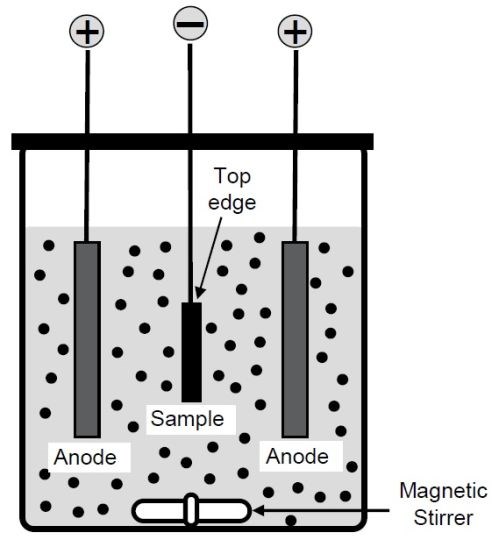
Figure 1 - Schematic of the electro-codeposition setup.
The solution was magnetically stirred at ~220 rpm, and the current density was varied from 30 to 200 mA/cm2. The specimens were plated for 30 to 180 min at 50°C. After plating, the specimen was rinsed and ultrasonically cleaned in hot water. The coatings were characterized by optical microscopy and scanning electron microscopy (SEM) equipped with energy dispersive spectroscopy (EDS). Prior to metallographic sample preparation, the specimens were copper-plated to improve edge retention. To determine the volume fraction of the incorporated CrAlY particles, multiple backscattered electron images were taken from different locations along the coating cross-section, which were then processed using the ImageJ software.18 Brightness and contrast of the images were adjusted by setting a proper threshold so that the particles could be separated from the background. The area fraction of the CrAlY particles was determined, which was assumed equivalent to its volume fraction.
IV. Results and discussion
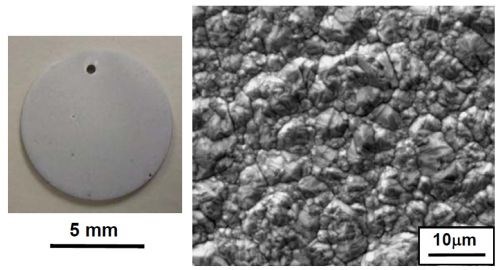
Uniform nickel coatings were plated with the fluoborate solution, as shown in Fig. 2. The nodules formed on the coating surface were not necessarily correlated to individual grains. Instead, they could be grain aggregates. The nickel coatings plated at 30 mA/cm2 for 60 min were 20-25 mm thick and were very adherent.
(a) (b)
(c)
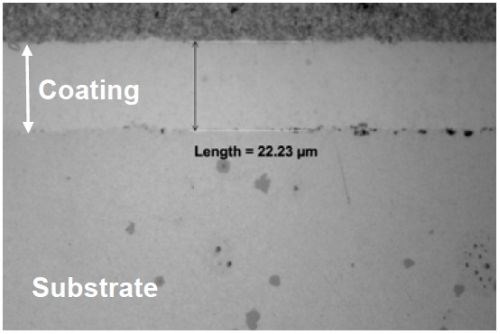
Figure 2 - Nickel coating plated at 30 mA/cm2 for 60 min using the fluoborate solution: (a) overall appearance; (b) SEM secondary electron image showing coating surface morphology; (c) optical micrograph of the coating cross section.
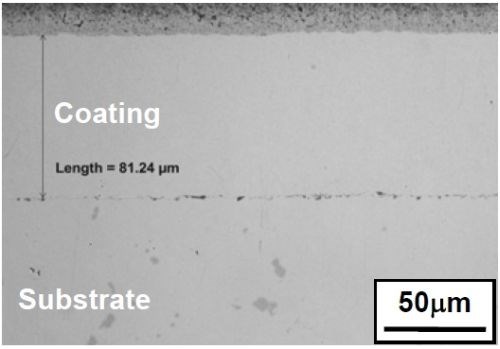
Nickel coatings were also deposited at higher current densities, e.g., 200 mA/cm2. As shown in Fig. 3, an adherent coating of ~80 mm thick was formed after 30-min plating. Nodules (thicker coatings) were observed on specimen edges, as indicated by the arrow in Fig. 3a. These results have further confirmed that the fluoborate bath has the capability of achieving high plating rates.
(a) (b)
(c)
Figure 3 - Nickel coating plated at 200 mA/cm2 for 30 min in the fluoborate solution: (a) overall appearance; (b) SEM secondary electron image showing coating surface morphology; (c) optical micrograph of the coating cross section.
Electro-codeposition experiments were also conducted in the fluoborate bath with the addition of CrAlY powder. However, when the powder was added, the fluoborate solution violently reacted with the powder. After the powder was thoroughly mixed with the solution, a significant drop of pH from 3.4 to ~2.0 was observed. Nickel carbonate was used to increase the pH of the plating solution to 2.5. Further solution pH modification was found difficult by adding more nickel carbonate. Since such a pH value was still in the recommended range for the fluoborate bath, the electro-codeposition was carried out at pH of 2.5. When the current was applied, small gas bubbles were noticed on both the cathode (specimen) and the anodes. Even with vigorous agitation, a uniform suspension could not form. Instead, several distinct layers were observed in the beaker.
(a) (b)
(c)
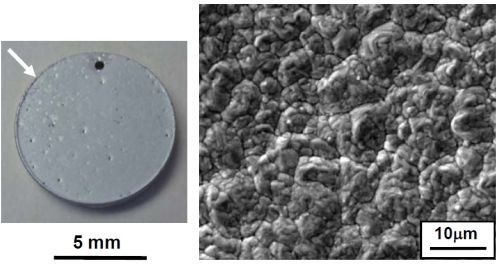
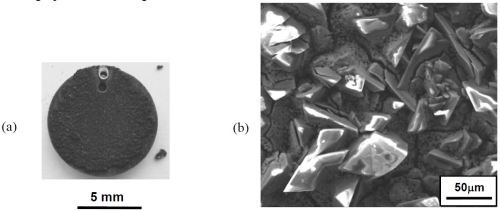
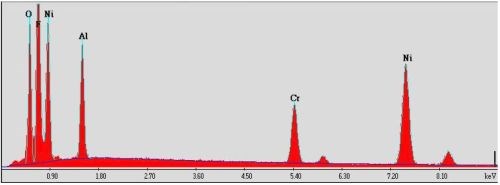
Figure 4 - Specimen plated in the fluoborate solution containing CrAlY powder: (a) overall appearance; (b) SEM secondary electron image showing coating surface morphology; (c) EDS analysis of the surface composition.
Figure 4 shows the specimen that was plated in the fluoborate solution containing 20 g/L of CrAlY powder. The specimen was plated under the same conditions as the one shown in Fig. 3 (i.e., at 200 mA/cm2 for 30 min), with the exception of the CrAlY powder in the solution. A dark powdery coating was formed on the specimen surface (Fig. 4a), which was loosely attached to the substrate. When the specimen was examined in the SEM, charging occurred (Fig. 4b), indicating that the coating surface exhibited poor electrical conductivity. Based on the EDS analysis in Fig. 4c, in addition to the expected elements of Ni, Cr and Al, other elements such as F and O were also detected on the coating surface. These impurities were introduced to the coating during electro-codeposition.
Although the fluoborate solution is sulfur-free and offers high plating rates, the fluoborate anion is aggressive. Some metallic materials that contact the solution can be chemically attacked, among which are aluminum, lead, titanium, and high silicon cast iron.12,19 In the electro-codeposition process, the CrAlY powder reacted with the fluoborate solution at 50°C and formed aluminum hydroxide, leading to the presence of the high oxygen peak on the specimen surface. Therefore, the fluoborate-based plating bath may be suitable for codeposition of more inert particles but not for relatively active metal particles such as the CrAlY-based powders. Future work will focus on the other sulfur-free bath, i.e., the all-chloride solution.
References
- G.W. Goward, Surf. Coat. Technol., 108-109, 73-79 (1998).
- A. Feuerstein, et al., J. Therm. Spray Technol., 17 (2), 199-213 (2008).
- C.T.J. Low, R.G.A. Wills and F.C. Walsh, Surf. Coat. Technol., 201 (1-2), 371-383 (2006).
- F.C. Walsh and C. Ponce de Leon, Trans. Inst. Metal Fin., 92 (2), 83-98 (2014).
- Y. Zhang, JOM, 67 (11), 2599-2607 (2015).
- B.L. Bates, J.C. Witman and Y. Zhang, Mater. Manuf. Process, 31 (9), 1232-1237 (2016).
- J.R. Nicholls, MRS Bull., 28 (9), 659-670 (2003).
- A.V. Put, D. Oquab and E. Péré, et al., Oxid. Met., 75 (5-6), 247-279 (2011).
- J.L. Smialek, JOM, 52 (1), 22-25 (2000).
- B.A. Pint, Oxid. Met., 45 (1-2), 1-37 (1996).
- N.S. Bornstein, M.A. DeCrescente and J.G. Smeggil, Mater. Sci. Eng., A120, 175-178 (1989).
- G.A. Di Bari, in Modern Electroplating, 5th Edition, Edited by M. Schlesinger and M. Paunovic, John Wiley & Sons, 2010; pp. 79-114.
- R. Oriňáková, et al., J. Appl. Electrochem., 36 (9), 957-972 (2006).
- R. Brugger, in Nickel Plating, 1st Ed., Clare o’ Molesey Ltd, Molesey, Surrey, 1970; pp. 46-47.
- H.M. Heiling, Metall., 14, 549-561 (1960).
- NPCS Board of Consultants & Engineers (Author), Electroplating, Anodizing & Metal Treatment Hand Book, Asia Pacific Business Press Inc., 2017; p. 518
- N.V. Parthasaradhy, Practical Electroplating Handbook, Prentice Hall, 1989; p. 183.
- C.A. Schneider, W.S. Rasband and K.W. Eliceiri, Nat. Methods, 9 (7), 671-675 (2012).
- G.A. Di Bari, in the ASM Handbook, Volume 5, Surface Engineering, published by ASM International, 1994; pp. 201-212.
Past project reports
1. Quarter 1 (January-March 2018): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 82 (12), 13 (September 2018); Full paper: http://short.pfonline.com/NASF18Sep1.
2. Quarter 2 (April-June 2018): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 83 (1), 13 (October 2018); Full paper: http://short.pfonline.com/NASF18Oct1.
3. Quarter 3 (July-September 2018): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 83 (3), 15 (December 2018); Full paper: http://short.pfonline.com/NASF18Dec1.
4. Quarter 4 (October-December 2018): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 83 (7), 11 (April 2019); Full paper: http://short.pfonline.com/NASF19Apr1.
5. Quarter 5 (January-March): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 83 (10), 11 (July 2019); Full paper: http://short.pfonline.com/NASF19Jul1.
6. Quarter 6 (April-June): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 84 (1), 17 (October 2019); Full paper: http://short.pfonline.com/NASF19Oct2.
7. Quarter 7 (July-September): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 84 (3), 16 (December 2019); Full paper: http://short.pfonline.com/NASF19Dec1.
8. Quarter 8 (October-December): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 84 (6), 11 (March 2020); Full paper: http://short.pfonline.com/NASF20Mar3.
About the authors

Dr. Ying Zhang is Professor of Mechanical Engineering at Tennessee Technological University, in Cookeville, Tennessee. She holds a B.S. in Physical Metallurgy from Yanshan University (China)(1990), an M.S. in Materials Science and Engineering from Shanghai University (China)(1993) and a Ph.D. in Materials Science and Engineering from the University of Tennessee (Knoxville)(1998). Her research interests are related to high-temperature protective coatings for gas turbine engine applications; materials synthesis via chemical vapor deposition, pack cementation and electrodeposition, and high-temperature oxidation and corrosion. She is the author of numerous papers in materials science and has mentored several Graduate and Post-Graduate scholars.

Mr. Brian L. Bates is a R&D Engineer in the Center for Manufacturing Research at Tennessee Technological University, in Cookeville, Tennessee. He holds B.S. (2002) and M.S. (2010) degrees in Mechanical Engineering from Tennessee Technological University. Mr. Bates also worked at Alcoa-Howmet Corporation (Morristown, Tennessee) and in the Alloy Behavior & Design group at Oak Ridge National Laboratory. Mr. Bates has been actively involved in many research projects on coating fabrication and evaluation.
*Corresponding author:
Dr. Ying Zhang, Professor
Department of Mechanical Engineering
Tennessee Technological University
Cookeville, TN 38505-0001
Tel: (931) 372-3265
Fax: (931) 372-6340
Email: yzhang@tntech.edu
Related Content
An Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreProducts Finishing Reveals 2023 Qualifying Top Shops
Each year PF conducts its Top Shops Benchmarking Survey, offering shops a tool to better understand their overall performance in the industry. The program also recognizes shops that meet a set of criteria to qualify as Top Shops.
Read MoreNanotechnology Start-up Develops Gold Plating Replacement
Ag-Nano System LLC introduces a new method of electroplating based on golden silver nanoparticles aimed at replacing gold plating used in electrical circuits.
Read MoreTroubleshooting Alkaline Zinc
One of the most common problems that can arise when plating with alkaline zinc is an imbalance of brightener in the solution. In this helpful Ask the Expert article, Chad Murphy of Columbia Chemical discusses how different zinc metal concentrations and brightener concentrations can impact efficiency.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More





















