Electrochemical Assessment of Sn-Pb Solderability
The 1991 AES Gold Medal Award was given to D.M. Tench and D.P. Anderson for the Best Paper appearing in Plating and Surface Finishing in 1990. Their work dealt with a very important concern in electronics finishing in the 1990s - testing for solderability and relating it to real world performance.
by
D.M. Tench and D.P. Anderson
Editor’s Note: Originally published as D.M. Tench and D.P. Anderson, Plating and Surface Finishing, 77 (8), 44-46 (1990), and was awarded the 1991 AES Gold Medal for Best Paper published in Plating and Surface Finishing in 1990. A printable PDF version is available by clicking HERE.
ABSTRACT
A sequential electrochemical reduction method has been developed for the quantitative analysis of surface oxides pertinent to Sn-Pb solderability. Analytical results from various aging treatments have been found to correlate with solderability as determined by the wetting balance method. Under some circumstances, electrochemical reduction also restored solderability degraded by steam aging. Preliminary results indicate that this extremely sensitive analytical method may also be used to detect the Cu-Sn intermetallic species that form at the Cu/Sn-Pb interface and affect solderability both directly and indirectly.
Introduction
The loss of solderability of printed wiring boards and component leads during storage is a major problem that costs the electronics industry millions of dollars each year. It is clear from numerous studies1 that oxidation of the Sn-Pb surface and underlying Cu-Sn intermetallic layers is involved, but the nature of the various oxides and their roles in the degradation process are obscure. However, since humid environments are known to greatly exacerbate the problem, it is clear that an electrochemical mechanism is operative. Therefore, the vacuum techniques typically employed for surface analysis may not be strictly applicable. Consequently, electrochemical methods permitting in situ quantitative analysis of oxides generated in the Cu-Sn-Pb system are preferred, and could also be more easily applied in a production environment for process control.
Electrochemical reduction in aqueous solutions has previously been applied to the analysis of oxides on tin2-5 and is a promising candidate for Sn-Pb surface analysis. This article concerns a chronopotentiometric reduction method, which is shown to provide results that correlate with Sn-Pb solderability.
Experimental details
The standard test specimen was a 1.5-mm-diameter hard copper wire, 2.5 cm in length, which was masked with Teflon heat-shrink tubing to expose a 1-cm long section with a rounded end. This section was first plated with 10 μm of copper from a standard non-additive pyrophosphate bath at 55°C, then with 12 μm of eutectic Sn-Pb from a standard fluoborate bath at room temperature. During plating, the wire cathode was rotated at 2000 rpm to control mass transport in the solution. A 60/40 Sn-Pb ratio was verified by atomic absorption analysis of specimens dissolved in acid solution, and by XRF. The Sn-Pb deposit was re-flowed in water soluble oil at 235°C for minimal time prior to use.
Electrochemical reduction was performed in a borate buffer (9.55 g/L sodium borate and 6.18 g/L boric acid) at a pH of 8.4 under an argon atmosphere in a 200 mL glass cell having separate compartments for the platinum counter electrode and reference saturated calomel electrode (SCE)(Fig. 1). All electrochemical experiments were performed using a potentiostat/ galvanostat.* Solderability tests were performed using a modified Wilhelmy wetting balance in conjunction with a digital oscilloscope.**

Figure 1 - Apparatus for performing sequential reduction analysis on actual printed circuit boards.
Results and discussion
Figure 2 shows chronopotentiograms that illustrate the sequential electrochemical reduction method for analysis of oxides on Sn-Pb surfaces. A constant current was applied in a borate buffer solution (pH 8.4) and the electrode potential vs. a reference electrode (SCE) was followed as a function of time. The electrodes were first mildly anodized (at 0.0 V) to oxidize the surface. Waves corresponding to reduction of a single oxide, (presumably PbO6), and two tin oxides, (presumably SnO and SnO26), were evident at -0.6., -0.9 and -1.1 V, respectively. As determined from the charge passed, all of these oxides were thin (1-3 monolayers). For eutectic Sn-Pb, no SnO2 was formed under the conditions employed. The small initial dip for tin suggests the presence of a duplex structure with a blocking layer of more stable oxide at the outer surface.
From cyclic voltammetric measurements involving pure lead and tin electrodes, there was a one-to-one correspondence between the anodic charge passed during formation of the oxides and the cathodic charge required for their reduction. This indicates that the electrochemical reduction method provides a quantitative measure of the amount of each surface oxide present. It should be mentioned, however, that similar chronopotentiometric experiments show that some tin dissolution occurs during prolonged anodization.

Figure 2 - Sequential electrochemical reduction analysis of the oxides formed on tin, lead and eutectic Sn-Pb under mildly oxidizing conditions.
Illustrative sequential reduction curves for eutectic Sn-Pb specimens subjected to more stringent oxidation treatments are reproduced in Fig. 3. A curve for a mildly anodized sample is included for comparison. Steam aging for 16 hr is seen to produce a much thicker oxide (on the order of 50 atom layers) comprised almost entirely of the higher oxide (SnO2). Strong anodization (passing 220 mC/cm2 of charge at 0.4 V) also produced a thick oxide that was not as well-defined and contained some SnO. The fact that such thick oxides can be sequentially reduced indicates appreciable oxide porosity. Strongly oxidized samples are consistently found to exhibit very poor solder wetting characteristics, thereby establishing a link between the higher tin oxides and solderability degradation.

Figure 3 - Sequential electrochemical reduction of oxides formed on eutectic Sn-Pb under strongly oxidizing conditions.
Electrolytic reduction has also been found to restore solderability lost as a result of the formation of Sn-Pb surface oxides. Figure 4 illustrates the dramatic improvement in wetting time and force attained (using a non-activated resin flux) for steam-aged eutectic Sn-Pb coatings by electrochemical reduction and by chemical reduction in 1.0M hydrazine solution.
As a means of investigating the nature of the principal intermetallic oxide, some of the standard copper-plated wire specimens were plated with 3 μm of tin, then heated under vacuum at 200°C for three days to convert all of the tin to Cu3Sn. Complete conversion was verified by SEM cross-sectional analysis. These specimens were found to be almost completely unwettable by solder. Figure 5 summarizes sequential stripping results for the Cu3Sn-coated specimens obtained in an argon-saturated borate buffer. The native oxide, which to some extent was formed at elevated temperature by reaction with residual oxygen, was ill-defined and about five atom layers thick. Apparently it can be completely reduced in this electrolyte. The reduced surface was stable in the deaerated solution, as evident from the curve obtained after 30 sec on open circuit. Exposure of the reduced surface to air for only 5 sec, however, resulted in regrowth of fiveatom layers of oxide that exhibited a well-defined plateau of about the same voltage as observed for SnO reduction (-0.9 V). Overnight, this oxide thickened to >10 atom layers, and the reduction curve obtained exhibited a long tail, indicating the presence of a more stable oxide. These results indicate that the major problem with the Cu3Sn intermetallic is the fast rate at which relatively thick oxide layers form on its surface. This may be a consequence of local cell action in which copper acts as the cathode.
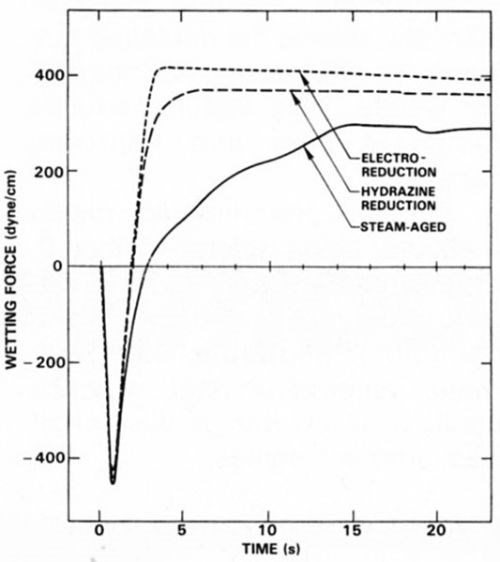
Figure 4 - Effect of electrochemical reduction in pH 8.4 borate buffer and chemical reduction in 1.0M hydrazine solution on the wetting balance behavior of steam-aged Sn-Pb specimens.
More work in progress
This method represents a powerful tool for assessing and understanding solderability degradation and may lead to a rapid test for determining the solderability state of production circuit boards. Note that the benign electrolyte used is compatible with PWB materials, and a small clip-on cell has been designed to assess solder ability anywhere along the board surface within through-holes. Work is still in progress on this method, so additional results can be expected.

Figure 5 - Sequential reduction analysis of the Cu-Sn native oxide and oxides formed after exposure to air.
References
1. R.J. Klein Wassink, Soldering in Electronics, Electrochemical Pub. Ltd., Ayr, Scotland, 1984; p. 98.
2. W. Katz, Stahl u. Eisen, 76, 1672 (1956).
3. F.W. Salt and J.G.N. Thomas, Nature, 178, 434 (1956).
4. S.C. Britton and K. Bright, Metallurgia, 56, 163 (1957).
5. R.P. Frankenthal, T.J. Butler and R.T. Davis Jr., Analytical Chem., 30, 441 (1958).
6. T. Farrell, Met. Sci., 10, 87, (Mar. 1976).
Footnotes
*PAR model 173, EG&G Princeton Applied Research Corp., Princeton, NJ.
**Nicolet model 2090. Nicolet Instrument Corp., Madison, WI.
About the authors


Related Content
How to Choose Between Sulfate and Chloride-Based Trivalent Chromium
There are several factors to consider when choosing between sulfate and chloride-based baths for trivalent chromium plating. Mark Schario of Columbia Chemical discusses the differences and what platers should keep in mind when evaluating options.
Read MoreNanotechnology Start-up Develops Gold Plating Replacement
Ag-Nano System LLC introduces a new method of electroplating based on golden silver nanoparticles aimed at replacing gold plating used in electrical circuits.
Read More3 Tests to Ensure Parts are Clean Prior to Plating
Making sure that all of the pre-processing fluids are removed prior to plating is not as simple as it seems. Rich Held of Haviland Products outlines three tests that can help verify that your parts are clean.
Read MoreTrivalent Chrome Overview
As the finishing industry begins to move away from the use of hexavalent chromium to trivalent chromium, what factors should finishers consider as they make new investments? Mark Schario, chief technology officer for Columbia Chemical offers a helpful overview of this complicated topic.
Read MoreRead Next
Episode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More




























