Electrochemical Destruction of Perfluorooctanesulfonate in Electroplating Wastewaters - 1st & 2nd Quarterly Report
This is the first report on a new NASF-AESF Foundation research project dealing with PFAS - a critical issue for the surface finishing industry. The project will study an electrochemical, destructive treatment strategy for the remediation of relevant PFASs in electroplating wastewater. The overall objective is to utilize a cost-effective reactive electrochemical membrane (REM) for the removal of PFAS from synthetic electroplating wastewater.
Part 1 - Introduction to the Project Work
1. Introduction and Significance to NASF
Per- and polyfluoroalkyl substances (PFASs) are a class of chemicals that have unique properties that impart oil and water repellency. As a result, PFASs have been used as coatings for textiles, paper products and consumer packaging, as well as for metal plating1 and in various other industries (e.g., semiconductors, automotive, construction).2 As a result of the widespread use of PFASs and their resistance to destructive treatment and biodegradation, these compounds are ubiquitous in the environment.3 There is growing evidence of their toxicity to humans.4 In response, the Environmental Protection Agency (EPA) has issued a combined Health Advisory Level for perfluorooctanoic acid (PFOA) and perfluorooctanesulfonate (PFOS) of 70 ng/L, and several states have endorsed standards for these and other PFASs.4 However, due to its ability to act as mist suppressant, PFOS has been used for metal finishing and electroplating applications in the form of tetraethylammonium PFOS salt (CAS #56773-42-3).5 New formulations have been introduced that utilize other polyfluoralkyl substances (e.g., 6:2 fluorotelomer sulfonate (6:2 FTS)) in place of PFOS. Given the health concerns and emerging regulatory standards of PFASs, reliable low-cost methods for destructive removal of PFOS and other PFASs are greatly needed.6
This project will study an electrochemical, destructive treatment strategy for the remediation of relevant PFASs in electroplating wastewater. The overall objective of this work is to utilize a cost-effective reactive electrochemical membrane (REM) for the removal of PFAS from synthetic electroplating wastewater. The REM is a patent-pending technology developed in our laboratory that utilizes a conductive ceramic electrode material with micron-sized pores to electrochemically oxidize or reduce contaminants in a flow-through operation. Specific technical objectives associated with the proposed work include:
- Development of REMs for destructive PFAS removal in synthetic electroplating wastewater.
- Determination of the optimal operational mode.
- Calculation of energy requirements for the REM-based system and compare to those determined for GAC adsorption and other technologies.
Achieving these objectives will provide the necessary data to determine if the REM system is competitive with other treatment options and thus will allow for the pursuit of further funding from industry and other funding agencies.
Specific technical questions and hypotheses to be tested are stated below. The specific tasks referenced are described in Section 3:
Question 1: Can adsorbent materials be added to REMs to produce next generation REMs with enhanced sorption capacities for PFAS?
Hypothesis 1: It is hypothesized that the addition of electrically-conductive adsorbent materials with documented high adsorption capacities for PFAS will allow for the production of REMs that can adsorb and oxidize PFAS in electroplating wastewater. The polarization of the REM as an anode will create electrostatic forces that will attract PFAS anions to sorption sites on the REM.
These electrostatic forces coupled to adsorbent incorporation into the REM matrix are hypothesized to increase PFAS adsorption capacities. REM synthesis and bench-scale optimization will be used to test hypothesis 1.
Question 2: What is the best mode of operation for optimal REM performance for PFAS removal?
Hypothesis 2: It is hypothesized that there is an optimal potential, flow rate and operational strategy for PFAS removal from electroplating wastewaters using the REM. Task 2a is dedicated to bench-scale optimization studies that will determine the optimal design variables and test several different operational strategies. The key operational challenges associated with REM performance in wastewater are associated with anode/cathode REM fouling by other organic compounds or cathode membrane fouling by redox active metals or mineral precipitants (e.g., CaCO3). Periodic offline anodic treatment (> 2.5VSHE) of fouled REMs will remove organic compounds,7 and periodic reverse polarity treatment of fouled cathodes (polarized as anodes) will remove metal and mineral precipitants at a low energy cost. Particle clogging may occur but can be eliminated using periodic backwash treatments. Fouling will also be assessed here.
Question 3: Will the REMs be a technically effective and cost-efficient remediation strategy for PFAS-containing electroplating wastewater?
Hypothesis 3: It is hypothesized that REMs can be synthesized at low costs, operate under low energy requirements, and will prove to be sufficiently stable so they will be both a technically effective and cost-efficient remediation strategy. The combination of experimental bench-scale testing (Tasks 2a) and operational energy calculations (Task 2b) will be used to test this hypothesis. Task 2b is dedicated to calculation of preliminary energy costs, and these estimates will be compared to other technologies for PFAS removal.
2. Related work
In the proposed work, we are investigating the technical and economic feasibility of using REMs for treatment of synthetic electroplating wastewater containing PFAS. A schematic of one possible configuration of a REM unit is shown in Fig. 1(a). The concentric porous cathode/anode tubes shown in the figure are made of solid Ti4O7 (25 mm cathode OD; 200 mm long; pore size ~1.0 μm; porosity ~30%). Depending on the treatment objective or the need to remove carbonate scale from the cathode, the anode and cathode can be switched by changing the polarity of the direct current (DC) voltage, without any physical manipulation of the device.
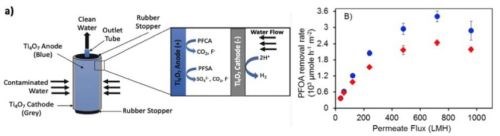
Figure 1 - (a) Schematic diagram of a concentric cylindrical reactive electrochemical membrane (REM) used for the treatment of perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkyl sulfonic acids (PFSAs) in liquid streams. The flow configuration is outside-in, dead end, filtration mode; (b) results for removal rates of PFOS (red diamonds) (3.3VSHE) and PFOA (blue circles) (2.9VSHE) oxidation in the REM as a function of permeate flux (electrolyte 100 mM K2HPO4). Starting concentrations for PFOA and PFOS were both 10 μM.8
The REM was used to degrade PFOA and PFOS in single-pass mode as a function of permeate flux (L/m2/hr (LMH)). The removal rates were calculated and are shown versus the permeate flux in Fig. 1(b). These results are very promising compared to other treatment methods. For example, at a permeate flux of 720 LMH the removal rates were 2436 and 3415 μmol/m2/hr for PFOA and PFOS, respectively.8 This permeate flux corresponded to a 3.8 sec residence time in the reactor and achieved 50 and 40% removal of PFOA and PFOS, respectively.8 Optimal energy consumption per log removal of 5.1 kWh/m3 for PFOA and 6.7 kWh/m3 for PFOS was achieved.8 The rate constants for PFOA and PFOS removal (607 and 210 per hr, respectively) in our work were over 100 times higher than previously reported for other treatment technologies, with energy consumptions that were a factor of ten lower.8
3. Technical approach
The work plan consists of REM synthesis, a series of bench-scale experimental studies that will determine optimal operating conditions for PFAS oxidation in synthetic electroplating wastewater samples, and a preliminary energy cost assessment. Experimental parameters that will be explored include: (1) adsorption capacity, (2) necessary residence time in the reactor, (3) needed membrane surface area per water volume treated and (4) energy usage (kWh/m3 water treated). The results will provide proof-of-concept that the REM technology is suitable for the treatment of PFOS in electroplating wastewater.
4. Relationship to NASF goals
This project is focused on the removal of PFAS from electroplating wastewater. There is little treatment of these wastewater streams at electroplating facilities, and typically these wastewaters are directly discharged to the municipal wastewater treatment plant. However, due to the growing concern over PFOS and other PFASs in the environment, there has been much further scrutiny of this practice by industries. A key example is in the state of Michigan, where regulators are considering imposing standards on industrial wastewaters containing PFASs (LINK HERE). The proposed work will focus on an economical and technically viable treatment solution, which can enable PFAS treatment. Therefore, it is expected that there would be considerable interest across fields in the electroplating community and beyond.
References
1. Z.W. Du, et al., “Efficient Adsorption of PFOS and F53B from Chrome Plating Wastewater and their Subsequent Degradation in the Regeneration Process,” Chem. Eng. J., 290, 405-413 (2016).
2. Interstate Technology and Regulatory Council, “History and Use of Per- and Polyfluoroalkyl Substances (PFAS),” (2017); https://pfas-1.itrcweb.org/wp-content/uploads/2017/11/pfas_fact_sheet_history_and_use__11_13_17.pdf (last accessed 09/10/2019).
3. I. Ross, et al., “A Review of Emerging Technologies for Remediation of PFASs,” Remediation Journal, 28 (2), 101-126 (2018); https://cswab.org/wp-content/uploads/2018/05/Emerging-Technologies-for-Remediation-of-PFAS-Ross-2018.pdf (last accessed 09/10/2019).
4. Interstate Technology and Regulatory Council, “Regulations, Guidance and Advisories for Per- and Polyfluoroalkyl Substances (PFAS),” (2018); https://pfas-1.itrcweb.org/wp- content/uploads/2018/01/pfas_fact_sheet_regulations__1_4_18.pdf (last accessed 09/10/2019).
5. Environmental Protection Agency, “PFOS Chromium Electroplater Study, U.S. Environmental Protection Agency, Region 5,” (2009): https://www.in.gov/idem/ctap/files/plating_chromium_pfos_study.pdf (last accessed 09/10/2019).
6. J.B. Weiss, et al., “PFAS Analysis in Water for the Global Monitoring Plan of the Stockholm Convention: Set-up and Guidelines for Monitoring,” UNEP Chem. Branch, (2016); https://wedocs.unep.org/bitstream/handle/20.500.11822/29682/Analisis_PFAS_En.pdf?sequence=1&isAllowed=y; pp. 1-26 (last accessed 09/10/2019).
7. Y. Jing, L. Guo and B.P. Chaplin, “Electrochemical Impedance Spectroscopy Study of Membrane Fouling and Electrochemical Regeneration at a Sub-Stoichiometric TiO2 reactive Electrochemical Membrane,” J. Membrane Sci., 510, 510-523 (2016).
8. T.X.H. Le, H. Haflich, A.D. Shah and B.P. Chaplin, “Energy-Efficient Electrochemical Oxidation of Perfluoroalkyl Substances Using a Ti4O7 Reactive Electrochemical Membrane Anode, Environmental Science & Technology Letters, 6, (8), 504-510 (2019).
Part 2 - 1st and 2nd Quarter Report (April-September 2019)
Objective
The overall objective of this work is to utilize a cost-effective reactive electrochemical membrane (REM) for the removal of PFAS from synthetic electroplating wastewater. The REM is a patent-pending technology that utilizes a conductive ceramic electrode material with micron-sized pores to electrochemically oxidize or reduce contaminants in a flow-through operation. Specific technical objectives associated with the proposed work include:
- Development of REMs for destructive PFAS removal in synthetic electroplating wastewater.
- Determination of the optimal operational mode.
- Calculation of energy requirements for the REM-based system and compare to those determined for GAC adsorption and other technologies.
Summary
Work was conducted on the synthesis of reactive electrochemical membranes (REMs), both with and without sorbent materials (Task 1), and preliminary PFOS oxidation tests in synthetic electroplating wastewater (Task 2). Experiments that were conducted in a complimentary SERDP-funded project (SERDP project # ER18-1491) indicated that the addition of carbonaceous materials (i.e., multiwalled carbon nanotubes (MWCNTs)) and powder activated carbon (PAC) significantly increased the sorption capacity of PFOA. However, the carbon content within the REMs was not electrochemically stable. Therefore, the use of carbon-Ti4O7 composite REMs for the simultaneous adsorption and oxidation of PFOS was determined to not be a feasible strategy for PFOS remediation. The use of carbon-free Ti4O7 REMs for PFOS oxidation were tested in a single-pass flow-through reactor with a liquid residence time of ~11 sec. Results indicated that PFOS (feed concentration of 40 μM) was degraded by 65% at 2.9VSHE and by 81% at 3.6VSHE. These results have established proof of concept that the REM is a viable technology for PFOS removal from electroplating wastewater. Future work will focus on optimizing the operating conditions, investigating the removal of PFOS from real electroplating wastewater, and testing the performance of the REM for the oxidation of PFOS replacements (e.g., 6:2 FTS, GenX).
Carbon-REM electrochemical stability
In a complimentary SERDP-funded project the electrochemical stability of the PAC-REM and MWCNT-REM materials were tested as a function of the applied anodic potential (SERDP project # ER18-1491). These experiments were conducted in a flow-through reactor and effluent solution from the reactor was collected as a function of potential. Results are summarized in Fig. 1. Results for the PAC-REM indicated that it began to oxidize at applied potential values higher than 0.5VSHE.
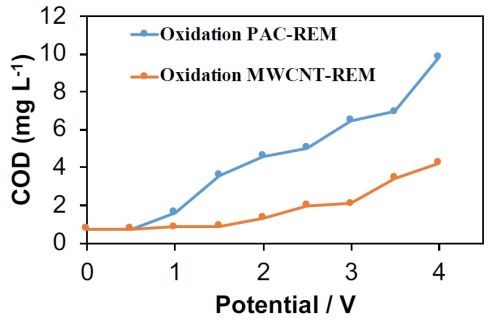
Figure 1 - COD permeate concentrations for PAC-REM and MWCNT-REM as a function of the anodic potential.
The COD value increased continuously from 0.76 mg/L at 0.5VSHE to 9.81 mg/L at 4.0VSHE. Results for the MWCNT-REM were more promising, but carbon leaching was still observed. For example, COD values in the effluent reached as high as 4.2 mg/L at an applied voltage of 4.0VSHE. These results indicated that the composite REMs were not stable under anodic conditions. To determine if the MWCNT-REM stability would improve with longer operation times, an additional experiment was conducted at 3.0VSHE over a four-hour period. Results are shown in Fig. 2 and indicate that stability of the MWCNT-REM deteriorated over time. Effluent COD levels were as high as 455 mg/L after 4 hours, which amounted to approximately 1.5% of the total carbon content. Thus, it is apparent that these composite REMs are not appropriate for PFAS oxidation. Therefore, other strategies are needed to remove and treat PFAS. Upstream adsorption followed by downstream oxidation is being explored.
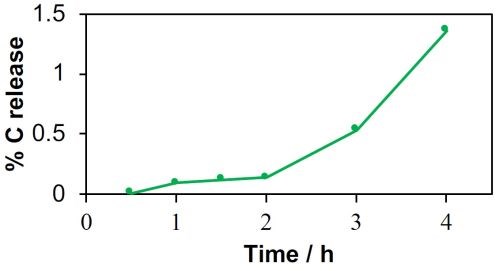
Figure 2 - Percent carbon leaching for the MWCNT-REM at a potential of 3.0 V/SHE as a function of time.
Electrostatic PFAS adsorption
Electrostatic adsorption of PFOA was investigated to determine if an adsorption step prior to electrochemical oxidation could be used to concentrate PFASs prior to electrochemical oxidation. Two flow-through electrode materials were investigated, including a Ti4O7 REM and a MWCNT-Ti4O7 REM. These REMs were polarized at an anodic potential of 0.5VSHE, which was a potential where the MWCNT-REM was stable. Results are shown in Figure 3 and indicate that the Ti4O7 REM did not provide any adsorption capacity. However, significant adsorption was observed for the MWCNT-REM. PFOA breakthrough occurred at over 2000 pore volumes and effluent concentrations did not reach influent concentration until approximately 10000 pore volumes. These results indicate that an electrosorption step prior to electrochemical oxidation could prove to be an effective treatment strategy for PFAS remediation. Ongoing work is focused on integrating the adsorption and oxidation steps.
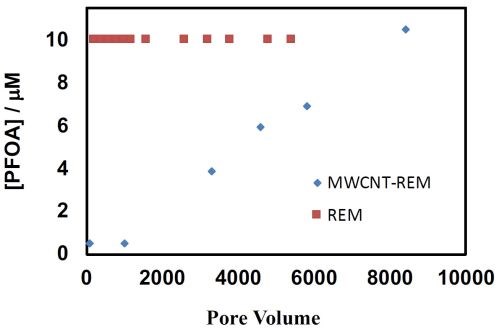
Figure 3 - PFOA effluent concentrations as a function of pore volumes processed in the REMs with an applied potential of 0.5VSHE. Influent PFOA concentration of 10 μM.
Electrochemical PFOS oxidation
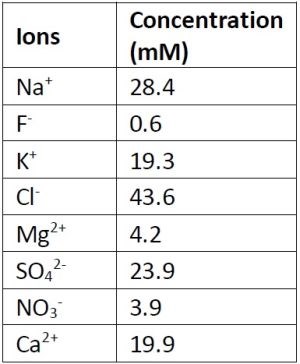
The electrochemical oxidation of 40 μM PFOS in a synthetic electroplating wastewater was tested using a Ti4O7 REM in a single-pass flow-through mode. The liquid residence time in the reactor was ~11 sec and oxidation was tested at anodic potentials of 2.9 and 3.5VSHE. The composition of the synthetic wastewater is listed in Table 1. Results indicated that PFOS was degraded by 65% at 2.9VSHE and 81% at 3.6VSHE. These results provide proof of concept that the REM is a viable technology for the elimination of PFOS from electroplating wastewater. Future work will focus on optimizing operating conditions, oxidation of real electroplating wastewaters, and investigation of the oxidation of PFOS replacements (e.g., 6:2 FTS, F-53B).
Table 1 - Composition of the synthetic electroplating waste-water. PFOS concentration was 40 μM.
Theoretical reactivity of PFOS replacements
Work has begun on determining the reactivity of PFOS replacements (e.g., 6:2 fluorotelomer sulfonate (6:2 FTS) and F-53B). Initial work focused on investigating the potential dependent reactivity of these compounds using density functional theory (DFT) modeling. A summary of these results is shown in Fig. 4, where the free energy of activation (∆G‡) versus the electrode potential is plotted for the direct oxidation reaction of 6:2 FTS, F-53B and PFOS. The direct oxidation reaction was modeled, as it has been shown to be the rate limiting step for PFOS oxidation. According to the results shown in Fig. 4, the reactivity of F-53B to the direct oxidation reaction should be similar to PFOS. However, results for 6:2 FTS show a much lower ∆G‡ value than both F-53B and PFOS at a given electrode potential. These results suggest that 6:2 FTS should react readily via electrochemical oxidation. Future work is planned to investigate the reactivity of both F-53B and 6:2 FTS.
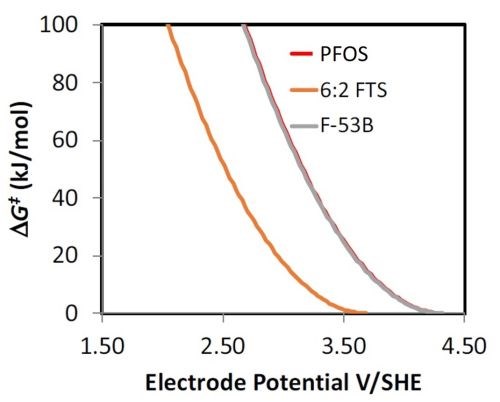
Figure 4 - DFT results showing the free energy of activation (∆G‡) versus the electrode potential for the direct oxidation reaction of 6:2 FTS, F-53B and PFOS.
About the author

Dr. Brian P. Chaplin is Associate Professor in the Department of Chemical Engineering, at the University of Illinois at Chicago. He holds a B. Civil Engineering (1999) and an M.S. (2003) in Civil Engineering from the University of Minnesota and a Ph.D. in Environmental Engineering (2007) from the University of Illinois at Urbana-Champaign.
* Dr. Brian Chaplin
Associate Professor
Dept. of Chemical Engineering
University of Illinois at Chicago
221 Chemical Engineering Building
810 S. Clinton St.
Chicago, IL 60607
Office: (312) 996-0288
Mobile: (217) 369-5529
E-mail: chaplin@uic.edu
Related Content
How to Maximize Nickel Plating Performance
The advantages of boric acid-free nickel plating include allowing manufacturers who utilize nickel plating to keep up the ever-changing regulatory policies and support sustainability efforts.
Read MoreTrivalent Chrome Overview
As the finishing industry begins to move away from the use of hexavalent chromium to trivalent chromium, what factors should finishers consider as they make new investments? Mark Schario, chief technology officer for Columbia Chemical offers a helpful overview of this complicated topic.
Read MoreAdvantages to Pumped Eductor Agitation
Not all agitation methods are created equally. Pumped agitation with eductor nozzles can improve process tanks and quickly show a reduction in operating costs while keeping staff safe, following environmental legislation and preventing pollution.
Read MoreAn Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More





















