Electroless Nickel: Beyond ELV
A look at the current state of EN technology
The term “electroless nickel” (EN) encompasses a broad class of coatings sharing the common attribute of being deposited electrochemically rather than utilizing an external source of electricity. Electroless nickel was discovered in 1944 by researchers at the U.S. National Bureau of Standards, who were trying to develop a new nickel-based electroplating solution and were investigating additives to reduce deposit stress.
By the 1980s, EN was firmly established. Since then, the technology has continued to evolve and expand into many different plating systems. Over the last 20 years or so, suppliers have refined process chemistry and improved our knowledge of the attributes and suitable applications of the various deposit types. Most of the development work on the technology has resulted in incremental improvements to deposit attributes, such as enhanced corrosion resistance, brightness, and hardness, as well as advances in the operational aspects of the processes, such as increased plating rate, better in-tank stability, and longer bath life.
Improved understanding of deposit attributes led to development of a classification system for the different types of EN plating. Detailed data on deposit attributes allowed design engineers, end users, applicators, and chemical suppliers to correctly choose and apply the coating with the most relevant attributes for a specific application.
Life was good…
The Present
Wham! The 21st century came, and with it came a North American surface finishing industry facing one of the most challenging business climates we’ve ever experienced. Migration of manufacturing to offshore locations has been exacerbated by unprecedented increases in raw material costs, notably nickel metal. Nickel metal prices over the last 5 years have increased astronomically, from a monthly average of $2.41/lb in December 2001 to $15.68/lb in December 2006.
A focus on environmental compliance has also significantly changed the way we approach our business. Among the most significant environmental drivers impacting the use of EN are the RoHS, WEEE, and ELV directives.
The Restriction of Hazardous Substances (RoHS, 2002/95/EC) and the Waste Electrical and Electronic Equipment (WEEE, 2002/96/EC) directives are intended to promote the reuse, recycling and recovery of electrical components. The European End of Life Vehicle Directive (ELV, 2000/53/EC) targets waste minimization through recycling and the elimination of hazardous waste from landfills.
RoHS restricts seven substances: lead, cadmium, mercury, hexavalent chrome, and polybrominated biphenyl (PBB) or polybrominated diphenyl ether (PBDE) flame retardants. It stipulates a maximum concentration value for these substances of 0.1 wt% (1,000 parts per million, or ppm) of lead, mercury, hexavalent chromium, PBB and PBDE and 0.01 wt% (100 ppm) cadmium in homogeneous materials.
ELV Annex II (9/20/05) permits the same concentrations of lead, hexavalent chromium, mercury and cadmium. Both documents consider a plated layer a homogeneous material.
In the United States, NSF/ANSI 51-2005 Food Equipment Materials, published in July of 2005 by NSF International, addresses the content of materials which may contact foodstuffs. It prohibits lead, arsenic, cadmium or mercury as intentional ingredients, and limits lead impurity in coatings to 0.06%.
The Japanese Green Procurement Survey Standard Initiative (JGPSSI) (July 2003) is another environmental driver. Driven by leading electronics companies such as Sony, Matsushita, Canon, and others, this policy contains lists of both regulated substances (Level A) and other substances (Level B) that are not regulated. The concern that at some point some of the materials on the Level B list may move to Level A status—and thus be subject to regulation—has led some Japanese companies to ask for EN coatings that contain no Level B substances. Several of the commonly used replacements in commercially available lead/cadmium free electroless nickel systems utilize materials that can be found in the JGPSSI Level B list.
Impact of Environmental Drivers
Both cadmium and lead have been used in EN solutions for many years to aid deposit brightness and provide process stability. The amount of lead co-deposited in the coating from hypophosphite-reduced EN formulations is normally below 0.06 wt%. In cadmium-brightened, hypophosphite-reduced systems, the amount of cadmium varies widely, but generally exceeds 0.05%, well over the 0.01% limit set by most of the above regulations.
Many commercially available conventional lead-stabilized processes meet some of the current environmental regulations. In particular, most existing lead-stabilized baths that do not contain cadmium will normally provide a deposit below ELV and RoHS limits for lead and cadmium. These systems would currently be suitable for use in applications requiring compliance to these regulations.
However, there is still a potential cause for concern. It is very possible that future legislation and/or OEM specifications will mandate the total elimination of lead and cadmium from electroless nickel deposits.
Adoption of these initiatives and regulations thus meant a significant change for the majority of EN chemistry suppliers for the impacted industries. While lead- and cadmium-free systems have been commercially available for decades, the vast majority of users would still need to change their EN process because the majority of processes used still contained lead and possibly cadmium.
Virtually all electroless nickel suppliers now offer environmentally compliant chemistries. Some of these systems have been specifically developed over the last several years to meet the regulations, while others were previously existing technology. These systems do not utilize either lead or cadmium, and can successfully replace traditional lead/cadmium containing chemistry in virtually all applications. They contain no lead or cadmium beyond trace impurities and thus satisfy the requirements of all the regulations.
But many of the materials utilized in these replacement chemistries are beginning to show up on “lists of concern”. Specifically, the Level B list of the JGPSSI contains several of the commonly used replacement materials. While this list does not in any way purport to a list of substances subject to future regulation, it is possible that some of the items on this list may become regulated materials.
Organic Additives
One avenue under development is to simply eliminate the substances in question and replace the metals commonly used as stabilizers and brighteners with organic compounds which serve the same function. These systems have been commercially available for years, and have performed well in field service. They represent the next generation in environmentally compliant EN technology, because they contain no metallic stabilizers or brighteners. The stabilizers and brighteners utilized in these chemistries are 100% compliant with RoHS, ELV, NSF, and JGPSSI Level A and Level B regulations. These systems eliminate any potential concern over future actions targeting metals contained in many current compliant systems, unless any future regulations target nickel metal itself.
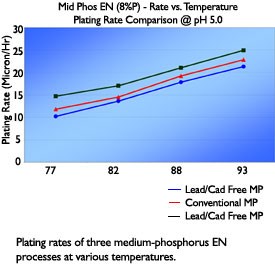
Multiple installations of these processes have shown that bath performance and deposit properties are comparable to both conventional EN as well as currently available compliant chemistry utilizing alternative metals in lieu of lead and cadmium. Plating rate, in-tank stability, deposit phosphorus content, hardness, and internal stress of deposits plated from the non-metal stabilized mid-phosphorus process have been very similar to conventional processes.
Traditionally, EN chemistries have been provided as three-component systems—typically, a common nickel component used in conjunction with both a dedicated component for make-up and a separate component for replenishment. The reason for this is that components of most metal-stabilized systems have potential precipitation issues. For example, most lead-stabilized processes do not mix the lead stabilizer together with the concentrated nickel sulfate component due to solubility issues.
Organic chemistries allow extreme flexibility in system format, both in component concentration and in allowing for a unique two-component system, where one component is added for make-up and one component is used for replenishment. This simplifies operation and reduces the number of components users need to maintain in inventory. Organically stabilized EN processes also offer potential operational advantages which will be discussed later.
Low-Metal Operation
There are a host of choices available to platers looking for environmentally compliant processes. However, what about the other drivers, such as the dramatic increase in nickel prices discussed earlier? One strategy to address this is to maximize the effective usage of the nickel-containing plating solution, and low-metal operation (LMO) has been used successfully for years to accomplish this.
In a normal plating operation, there is some drag-out during processing. Losses vary widely based on part geometry, racking method, rack vs. barrel operation, hang time after plating, rinsing strategy, surface tension of the plating solution, and other factors. If the plating process operates at half the metal concentration of a conventional EN bath, then drag-out losses will automatically be cut in half. This can be a significant source of loss in some plating facilities, and at current nickel prices any degree of loss has an impact on the bottom line.
At the end of useful bath life, the process is usually discarded and a new solution made up. In a conventional system running at 6 g/L of nickel, the loss of metal can be significant. Even plating the process down before dumping only partially mitigates the loss, because the typical plate-down point is somewhere between 100% and about 70% activity, or 6.0 to 4.2 g/L.
By comparison, a LMO bath is dumped at 3 g/L maximum. At a cost of $16/lb of nickel metal, savings can be as high as $0.40/gal of spent solution on metal alone, not counting the impact of other bath constituents or waste treatment costs.
A typical LMO process will plate 5 to 10% more area before dumping than a conventional EN chemistry. At makeup, a standard hypophosphite-reduced EN bath running at 6 g/L of nickel will contain about 120 g/L of dissolved solids. Each metal turnover (MTO) of operation adds 45 to 60 g/L of solids, depending on the bath formula. In a plating bath designed to run at 3 g/L of nickel, we can reduce the solids on makeup by 45 g/L less than a bath operating at 6 g/L. The reduction of dissolved solids on make-up allows the solution to hold more reaction by-products, resulting in a bath life extension of one-half to one MTO.
While LMO offers significant advantages in minimizing waste, there is a consequence of operating the bath at low strength. LMO baths can have a fairly narrow operating window with respect to bath concentration when compared to conventional electroless nickel systems. This is due to the reduced level of metallic stabilizers present in a LMO system.
Metallic stabilizers are commonly present in an EN process in ppm quantities, and these metals are co-deposited with the nickel during plating. In an LMO bath, the quantity of stabilizers is low to begin with, and if allowed to drop below a critical point can result in bath instability. For this reason, LMO processes have been utilized virtually exclusively in facilities with automated controls or in plants with consistent, predictable work loads, which allows for tight maintenance of bath concentration.
One aspect of the organically stabilized chemistries discussed earlier is a greater tolerance to both the absolute amount of stabilizer and the ratio of stabilizer to nickel. What this means operationally is that the need for tight control seen with metallically stabilized LMO processes is alleviated to the point where conventional maintenance of the LMO process becomes a viable option. By running organically stabilized EN as a LMO bath, we create a system that addresses two of the most significant issues facing today’s metal finishing professional by offering total environmental compliance combined with long term cost reductions.
The future is waiting. Are you ready?
Acknowledgements:
The author wishes to thank David Crotty for his irreplaceable assistance in the creation of this article, and also Duncan Beckett and Mark Jankowski for their invaluable input and suggestions.
Related Content
Top 5 Areas to Consider Automation of Plating Operations
Automation for finishing operations can lead to improvements in process time, repeatability and consistency of quality. Yet, processes that make sense to explore for these operational efficiencies may not always be readily apparent.
Read MoreNASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 7th Quarterly Report
The NASF-AESF Foundation Research Board has selected a project on addressing the problem of PFAS and related chemicals in plating wastewater streams, studying PFAS destruction via electrooxidation and electrocoagulation. Our last report described the results from experiments of EO with a Magnéli phase Ti4O7 anode on the degradation of eight perfluoroalkyl acids (PFAAs). In this seven quarter report, we describe work to further explore how the degradation of different PFAAs are related to their molecular structures.
Read MoreUltrafiltration Membranes, Filter Elements for Improved Industrial Water Reuse
Ultrafiltration membranes help with water reuse in a variety of applications.
Read MoreNASF/AESF Foundation Research Project #120: Electrochemical Destruction of Perfluorooctanesulfonate in Electroplating Wastewaters – January – December 2023
This NASF-AESF Foundation research project report covers quarterly reporting for the year 2023 at the University of Illinois at Chicago. The objective of this work is to utilize a cost-effective reactive electrochemical membrane (REM) for the removal of PFAS from synthetic electroplating wastewater. Discussed here are the oxidation of PFOA with three different catalysts, development of a method for detecting PFAS, as well as work on 6:2-fluorotelomersulfonic acid (6:2 FTS) and electrodeposited bismuth/tin oxide catalysts.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More













.jpg;maxWidth=300;quality=90)









