Estimating the Total Solids Burden during the Waste Water Treatment of Vibratory Finishing Effluents
by William P.
by William P. Nebiolo
REM Surface Engineering, Southington, Conn.
During generic vibratory processing operations, abrasive ceramic media is utilized to abrade surface roughness from the parts to generate a smoother, functional or more attractive microfinish. Chelating and/or sequestering agents are commonly employed chemical intermediates in assorted proprietary vibratory processing compounds. Their role is keep the abrasive media clean by coupling with hard water salts or the metal swarf previously removed from the parts being processed. Without the chelants, loose metallic debris in the vibratory bowl will rapidly glaze-over the media and shut down its ability to abrade and thereby refine.
Local waste treatment regulations require that dissolved metals be separated from the collected effluent prior to its discharge from the manufacturing plant. Frequently, but not always, this technology is automated and requires the use of assorted acids and alkalis to accomplish the reaction wherein the metals are precipitated as metal hydroxides.
When designing/sizing a waste treatment and metal hydroxide collection system, it is essential to estimate the solids burden that will be generated. This will allow the manufacturing facility to project the volume of waste treatment chemistries that will be required to complete the precipitation work and also to estimate the size of the tankage and filter press that will be required during hydroxide precipitation and separation. Once these volumes have been calculated, it is then possible to anticipate the volume of filter cake that will be produced so plausible final solids disposal options can be reviewed.
This paper will review generically the traditional metal hydroxide separation techniques used for vibratory processes with special attention paid to the depth of metal removed from the parts being processed. Additionally, the effect of chelating agents in complicating the precipitation reaction as well as media attrition and its contribution to the total eventual solids burden will be reviewed. Since steel and abrasive ceramic media are the most common metal and media type that are utilized during vibratory finishing they will be the focus of this paper.
Additionally, to prevent media glazing, a vibratory processing fluid is added to the vibratory bowl.3 Chelants in the processing fluid dissolve metal removed from the parts. Since the dissolved metals must be separated from the waste water prior to final discharge, waste treatment chemicals must be added to break the chelation. The presence of the chelants then becomes of necessity a source of solids during waste treatment.
As the surface of the parts is abraded smooth the metals removed from the parts become a third source of solids to the waste treatment department.3
The final contribution of solids to the waste treatment department, comes from the waste treatment chemicals that must be utilized to break the chelation reaction and to precipitate the previously dissolved metals as insoluble hydroxides.3
Therefore, sources of vibratory process solids include:
• Media attrition and resultant swarf
• Processing liquid chelants
• Metal removed from the parts
• Waste treatment chemicals
Media bond number | % Porcelain binder | % Al2O3 abrasive | Media densitya, lb/ft3 |
High density | 0 | 100b | 125 |
0 | 100c | 0 | 90 |
10 | 92 | 8 | 88 |
20 | 85 | 15 | 85 |
30 | 75 | 25 | 82 |
40 | 65 | 35 | 80 |
50 | 55 | 45 | 78 |
60 | 40 | 60 | 75 |
70 | 25 | 75 | 72 |
Al2O3d | 0 | 100 | 70 |
aBased upon author’s experience bVitrified, 100% aluminum oxide media cPure porcelain binder, no Al2O3 abrasive d100% mineral Al2O3, non-kiln fired/non-vitrified | |||
Media bond number | Attrition ratea, lb/hr | Swarf, lb/hrb | Lb/hr swarf in a 20 ft3 vibratory bowlc |
High densityd | 0.008 | 0.080 | 0.133 |
0 | 0.100 | 1.000 | 1.152 |
10 | 0.130 | 1.300 | 1.464 |
20 | 0.350 | 3.500 | 3.808 |
30 | 0.750 | 7.500 | 7.872 |
40 | 1.200 | 12.00 | 12.29 |
50 | 2.000 | 20.00 | 19.97 |
60 | 2.750 | 27.50 | 26.40 |
70 | 3.500 | 35.00 | 32.26 |
XA | 4.000 | 40.00 | 35.84 |
aBased upon author’s experience and measurements bAssumes 1,000 lb. media in vibratory bowl at t = 0 c20 ft3 vibratory bowl with 16 ft3 usable volume and 12.8 ft3 media dHigh-density, vitrified, aluminum oxide media | |||
• Media = (16 ft3)(0.8 usage) = 12.8 ft3 media
• Parts = (16 ft3)(0.2 usage) = 3.2 ft3 parts
Now let us make the following additional assumptions for this example:
• The media is a 20-bond abrasive ceramic
• The processing time is two hours
Using the data found in Tables 1 and 2, it is possible to determine the actual weight of media consumed and therefore the weight of media sludge generated for the two-hour processing cycle as follows:
• From Table 1, 20-bond media has a weight density of 85 lb./ft3
• From Table 2, 20-bond media has an attrition rate of 0.35%/hr.
A two-hour processing cycle will generate the following weight of media sludge:
(12.8 ft3)(85 lb./ft3)(2 hr)(0.0035/hr) = 7.62 lb.
During the processing cycle the media becomes a source of abrasive swarf that is utilized to mechanically abrade the parts to a smooth finish.6 In this example, 8.27 lb. of abrasive swarf is generated in the two hours of vibratory bowl processing time and becomes a solids contributor to the vibe bowl effluent and the waste treatment system.
Depth of metal removal as a solids contributor
The purpose of the vibratory processing operation is of course to refine the surface of the parts loaded into the machine and/or to generate appropriate edge deburring properly. To complete this task, metal must be abraded from the surface of the parts being processed. The metal, coupled by the chelants, must be precipitated as an insoluble hydroxide and therefore contributes to the solids loading.6 It is possible to calculate the contribution of this metal as solids sent to the waste treatment department, as long as the depth of steel removed and the total surface area of steel loaded into the bowl is known. The following paragraphs highlight an illustrative example.
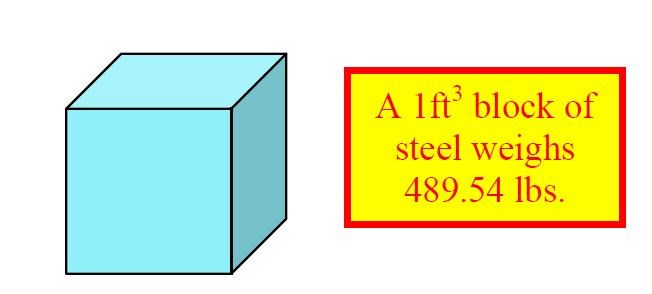
Consider first a one-cubic foot block of steel (Fig. 5), which weighs 489.54 lb.4,6
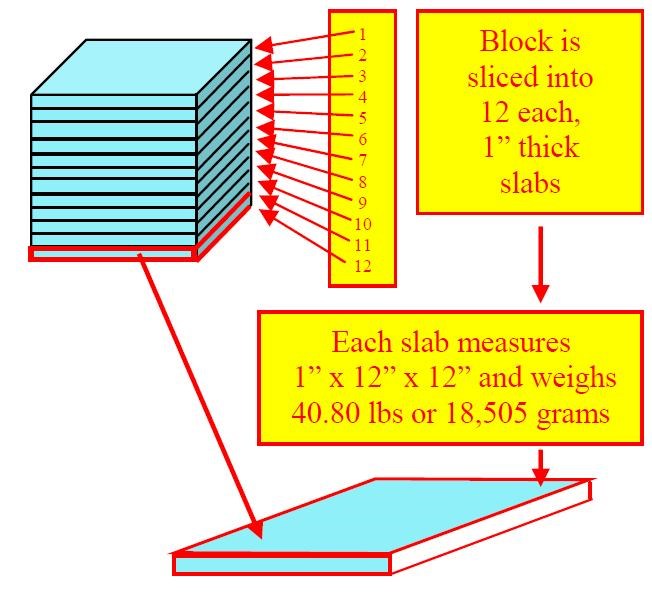
If this block of steel could be segmented into 12 uniform slabs, each slab would be one inch thick and would weigh 1/12th of the original 489.54 lb. or 40.80 lb. (Fig. 6).6
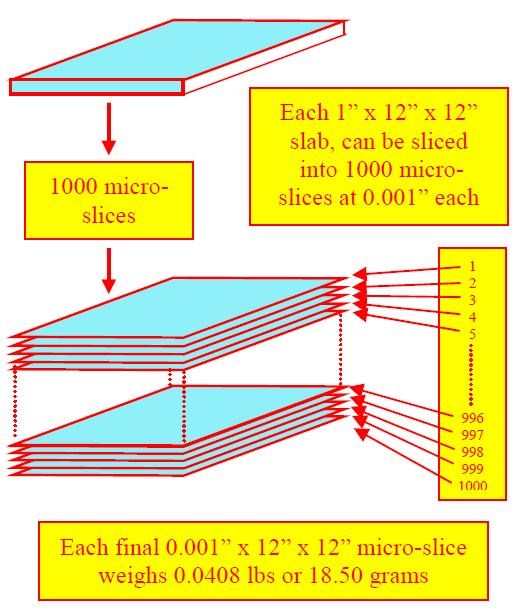
During traditional vibratory finishing however, metal removal occurs in thousandths of an inch depths. Knowing this, it is possible to additionally extrapolate the weight of steel that will be removed. If each 1 × 12 × 12-in. slab were now micro-sliced into one thousand individual sliver-thin slices, then each slice would weigh 1/1000 of 40.80 lb. or 0.0408 lb. (Fig. 7).6
Knowing this data, it is now possible to calculate the weight of steel that is removed from the parts during the vibratory processing cycle. Let us assume that a lot of steel parts with a total surface area of 50 ft2 is loaded into a vibratory bowl. The parts are processed to remove a depth of 0.0005 in. of steel. The solids loading of steel can be calculated as follows:
(50 ft2)(0.0408 lb./ft2/0.001”) ÷ 2 = 1.02 lb.
To make these calculations easier, Table 3 is a quick reference guide for the weight in lb. of steel solids removed from assorted surface areas of steel for assorted depths of metal removal.6
Steel Area, ft2 | Solids in lb., if 0.0001” removed | Solids in lb., if 0.0002” removed | Solids in lb., if 0.0005” removed |
1 | 0.00408 | 0.00816 | 0.02040 |
5 | 0.02040 | 0.04080 | 0.10200 |
10 | 0.04080 | 0.08160 | 0.20400 |
20 | 0.08160 | 0.16320 | 0.40800 |
25 | 0.10200 | 0.20400 | 0.51000 |
50 | 0.20400 | 0.40800 | 1.02000 |
100 | 0.40800 | 0.81600 | 2.04000 |
125 | 0.51000 | 1.02000 | 2.55000 |
• Sulfuric acid, H2SO4
• Calcium chloride, CaCl2
• Sodium hydroxide, NaOH
• Lime, Ca(OH)2
These chemistries are typically employed as they are the most inexpensive materials available in bulk, which can be utilized for pH neutralization and hydroxide precipitation.
During chemical reactions with the chelated metals, these chemicals are used to liberate the chelated cationic metals. This additionally results in the fact that the former non-needed cationic or anionic portion of the waste treatment chemical itself becomes a useless component in the reaction and an additional solids loading burden. This additional solids burden contributes to the total eventual solids loading based upon each molecule’s gravimetric factor.4,6
To better understand the beneficial portion of each molecule versus its pure gravimetric equivalent weight in solids burden, each of the chemicals is reviewed below. But first, a discussion on gravimetric equivalency follows.
Gravimetric equivalency and its relevance to waste treatment
Let us assume you have one pound of iron phosphate that is dissolved in water and you desire to precipitate the iron out of solution. The actual weight of the total iron phosphate molecule consists of the weight of the cationic (+ charged) iron ion plus the weight of the anionic (- charged) phosphate ion. The iron, which is the target for precipitation, is therefore just a small percentage of the total molecule’s weight.
Using the Periodic Chart of the Elements, it is possible to calculate the gram atomic weight (GAW) of the iron phosphate molecule from which the weight percent of iron can then be determined:
1. Iron phosphate has the molecular structure of FePO4. It contains one iron atom, one phosphorous atom and four oxygen atoms.
2. One atom of Fe = 55.85 g × 1 = 55.85 g
3. One atom of P = 30.97 g × 1 = 30.97 g
4. One atom of O = 16.00 g × 4 = 64.00 g
5. One GAW FePO4 = Total = 147.82 g
6. Percentage of iron in iron phosphate is:
(55.85)(100) ÷ 147.82 = 37.78% iron
7. Therefore, one pound of FePO4 contains:
a. 0.3778 lb. Fe+3 (iron cation)
b. 0.6222 lb. PO4-3 (phosphate anion)
Understanding the solids burden this represents, we now realize that when the waste treatment operator adds sufficient waste treatment chemicals to precipitate the 0.3778 lb. of iron, the 0.6222 lb. of phosphate will additionally become part of the precipitated solids weight. This ratio of the difference caused by the need to balance the molecular charges is known as gravimetric equivalency and for heavy metal-containing molecules, it can add considerable additional solids weight to the waste treatment burden.
1. A 20-ft3 vibratory bowl is used that has 16 ft3 of usable capacity.
2. The loading ratio of parts to media is 20% parts to 80% media.
3. The media that is being used is a 20-bond media.
4. The vibratory finishing department processes the parts for two hours.
5. The area of steel parts in the bowl is 50 ft2.
6. Processing removes 0.0005 in. of steel.
7. The waste treatment procedure at the facility treating the vibratory finishing effluent proceeds as follows:
a. Lower pH to 2.0 to cleave the heavy metals from the chelants.
b. Add calcium chloride to couple with the liberated chelants.
c. Raise the pH to 7.0 with sodium hydroxide.
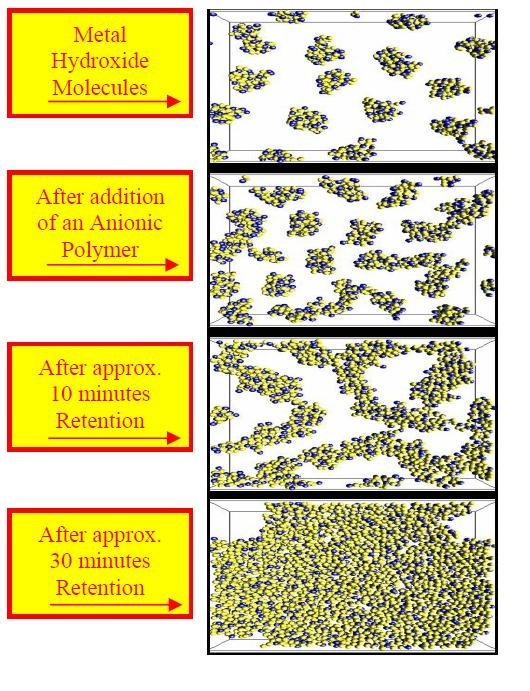
d. Add anionic polyelectrolyte to flocculate generated hydroxides.
e. Elevate the pH to 10 with lime to maximize hydroxide precipitation.
Steel solids = 1.02 lb.
Media solids = 7.62 lb.
Sulfuric acid = 1.07 lb.
Calcium chloride = 1.80 lb.
Sodium hydroxide = 1.94 lb.
Lime = 2.40 lb.
Total Solids = 15.85 lb.
• Final filter cake solids weight for each pound of steel removed
(15.85 lb.)(100) ÷ 35 = 45.29 lb. moist cake
• Final filter cake solids volume for each pound of steel removed
45.29 lb. moist cake ÷ 80 lb./ft3 = 0.57 ft3
As can be seen from the previous calculations, one pound of steel (iron) that is initially dissolved into solution during vibratory processing can result in a substantial volume of final solids.6
The example illustrated in this paper doesn’t necessarily apply just to vibratory finishing. Iron in any waste water will require similar waste treatment, precipitation and separation techniques. Therefore the factors present in this paper can be used for other waste treatment calculations.
Table 4 details the expected filter cake solids weight and filter cake volume generated for the data presented in Table 3.
Steel depth | Final solids | Area, ft2 1 | Area, ft2 5 | Area, ft2 10 |
0.0001 in. | Lb. solids | 0.03 | 0.16 | 0.33 |
Lb. cake | 0.09 | 0.46 | 0.94 | |
Ft3 cake | 0.001 | 0.006 | 0.012 | |
0.0002 in. | Lb. solids | 0.07 | 0.33 | 0.66 |
Lb. cake | 0.19 | 0.94 | 1.89 | |
Ft3 cake | 0.002 | 0.012 | 0.024 | |
0.0004 in. | Lb. solids | 0.13 | 0.67 | 1.32 |
Lb. cake | 0.38 | 1.91 | 3.77 | |
Ft3 cake | 0.004 | 0.024 | 0.047 | |
0.0005 in. | Lb. solids | 0.17 | 0.82 | 1.65 |
Lb. cake | 0.43 | 2.34 | 4.71 | |
Ft3 cake | 0.005 | 0.290 | 0.059 | |
0.0006 in. | Lb. solids | 0.20 | 0.98 | 1.98 |
Lb. cake | 0.57 | 2.80 | 5.66 | |
Ft3 cake | 0.007 | 0.035 | 0.071 | |
0.0008 in. | Lb. solids | 0.26 | 1.34 | 2.64 |
Lb. cake | 0.76 | 3.83 | 7.54 | |
Ft3 cake | 0.010 | 0.048 | 0.094 | |
0.0010 in. | Lb. solids | 0.33 | 1.64 | 3.30 |
Lb. cake | 0.86 | 4.69 | 9.43 | |
Ft3 cake | 0.011 | 0.059 | 0.118 |
As an example, assume 0.0005 in. of steel has been removed from 10 ft2 of steel parts. Moving across Row 5 for 0.0005 in. removed to Column 5 for 10 ft2 of steel area, you will:
• generate 1.65 lb. of hydroxide solids which when filter pressed,
• yield 4.71 lb. of moist filter cake solids, that
• occupy 0.059 ft3 of volume.
• Media swarf for media attrition
• Metal removed from the parts
• Hydroxides created during the waste treatment precipitation step
• Gravimetric additional weights from the contaminants and the added waste treatment chemistries that are non-reactive ingredients present in the original molecules
Using media of a lower bond number results in less media solids to waste treatment because lower bond media has a higher concentration of ceramic binder.
Metal removed from the parts is frequently chelated by the vibratory processing liquids to prevent media glazing. These chelants must be deactivated during waste treatment by the intentional addition of calcium. Using a non-chelated burnish compound can eliminate this step thereby reducing solids loading.
- W.P. Nebiolo, Proc. SUR/FIN 2005, St. Louis, MO, NASF, Washington, DC, 2005; pp. 605-620.
- W.P. Nebiolo, Proc. SUR/FIN 2008, Indianapolis, IN, NASF, Washington, DC, 2008; p. 53.
- W.P. Nebiolo, REM Training Manual, 5th Edition, REM Surface Engineering, Southington, CT, 2011.
- W.P. Nebiolo, REM Standards and Factors, REM Surface Engineering, Southington, CT, 2010.
- W.P. Nebiolo, Proc. SUR/FIN 2010, Grand Rapids, MI, NASF, Washington, DC, 2010; also www.pfonline.com/articles/increasing-vibratory-efficiency-by-optimizing-the-choice-of-media-shape-relative-to-part-geometry.
- W.P. Nebiolo, REM General Waste Treatment Procedures, REM Surface Engineering, Southington, CT, 2010.
Related Content
Highlights from SUR/FIN 2023
Products Finishing offers a recap of some of the topics that were top of mind at the SUR/FIN 2023 finishing industry trade show.
Read MoreTin-Zinc Alloy Electroplating and Its Corrosion Behavior
An NASF/AESF Foundation Research Program Retrospective
Read MorePlasma Electrolytic Oxidation (PEO): A High-Performance Coating for Light Metal Alloys
Plasma Electrolytic Oxidation (PEO) offers an innovative approach to high-performance coatings for light metal alloys, providing superior alternatives to traditional hard anodizing. The process transforms the surface of metals like Al, Mg and Ti into a robust oxide layer with customizable properties, tailored for demanding applications in aerospace, semiconductor, and industrial manufacturing.
Read MoreSUR/FIN 2023 Registration Is Now Open
The National Association for Surface Finishing SUR/FIN 2023 surface finishing industry trade show will take place June 6-8, 2023 in Cleveland, Ohio.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More











.jpg;maxWidth=600)










.jpg;maxWidth=300;quality=90)








