HPLC Study on Copper Plating Chemistry
In a typical acid copper plating bath, additives, such as grain refiners, brighteners and carriers, are used to deliver a smooth, bright and hard deposit. In order to ensure high quality plating, it is helpful to have a better understanding of the behavior of these additives in the bath. In this paper, a high performance liquid chromatography (HPLC) study of these additives is discussed, in an attempt to answer the following questions: Do these additives exist in the solution as a complex mixture of derivatives? Are there interactions between them? Are there any decomposition species accumulated in the plating process? How can we better control the plating process? The results indicate that the brightener exists in the bath with a series of its derivatives, while the grain refiner is in a simple form. When the brightener and the grain refiner were mixed in solution, some new species, which have different retention times in HPLC, were generated. Combined with a Hull Cell study, the impacts of the new species and the concentrations of additives on the plating process were investigated. Decomposition of the plating solution was also examined.
Introduction
Electrodeposition of copper in an acid bath has been widely used for various industrial applications. It is well known that additives can be used in the bath as grain refiners, brighteners and carriers to produce copper with a smooth and bright surface. Chemical reactions of these additives in the plating bath have significant effects on the quality of the deposit. From time to time, people have questioned how stable a bright copper bath is, whether the additives change when there is no current passed in the bath, or whether there is a better way to control the changes of additives during plating. Having answers to these questions is important because the plating process can easily drift out of control if the bath chemistry is not well understood. In this paper, high performance liquid chromatography (HPLC) and Hull Cell results are reported on the additive behavior in an acid bath. The interactions between different additives have been found, the impacts on the stabilities of the additives revealed, and the mechanism for these reactions suggested.
Experimental
The HPLC study was run on a Dionex 4500i HPLC system, with an Ionpac CS10 Analytical Ion Exchange Column and a UV detector. Eluent A was 27 mM of sulfuric acid and Eluent B was 50% acetone nitride and 50% 27 mM sulfuric acid. The gradient was as follows:
- 00.0 min: 2% of Eluent B
- 20.0 min: 80% of Eluent B
- 25.0 min: 2% of Eluent B.
Hull Cell experiments were run in a 267-mL cell. A brass panel was used as the cathode and copper pellets as the anode. A current of 3.0 A was passed for five minutes.
The experiments were conducted in a typical acid copper bath. The basic solution is composed of copper sulfate and sulfuric acid. Commercially available chemicals were used as the samples for HPLC and Hull Cell study. The proprietary grain refiner, brightener and carrier were purchased from a chemical process supplier.
HPLC of additives
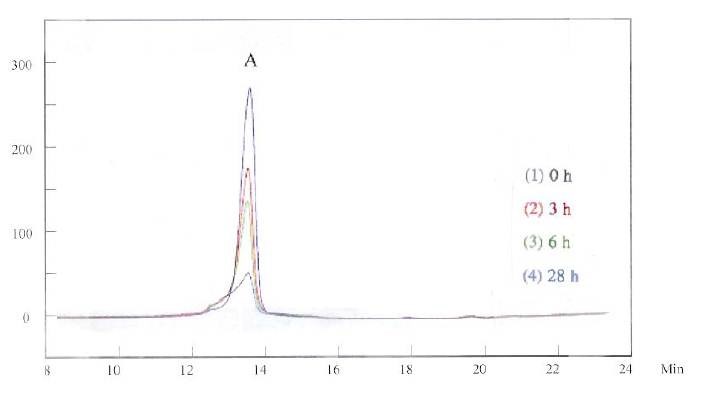
Figure 1 shows the HPLC curves for the grain refiner in the basic solution. The first HPLC measurement was conducted immediately after the grain refiner was mixed with the basic copper solution, and is recorded as “0 h.” The measurements were conducted and then taken three, six and 28 hours later, recorded as “3 h,” “6 h” and “28 h,” respectively. The x-axis in Fig. 1 is the retention time in minutes. The y-axis in the figure shows the relative intensity of the detected signal in millivolts. The HPLC was detected at 215 nm by the UV detector. Figure 1 shows that the grain refiner has a HPLC peak at about 13.5 min (Peak A) under this condition. It is not stable in the copper plating solution. After 28 hr, only one-fifth of intensity was detected.
When the HPLC was operated at a detection wavelength of 245 nm, a similar but larger Peak A was recorded, shown in Fig. 2. While HPLC did not show much change at the two different wavelengths for the grain refiner, it did make difference for the brightener, as discussed next.
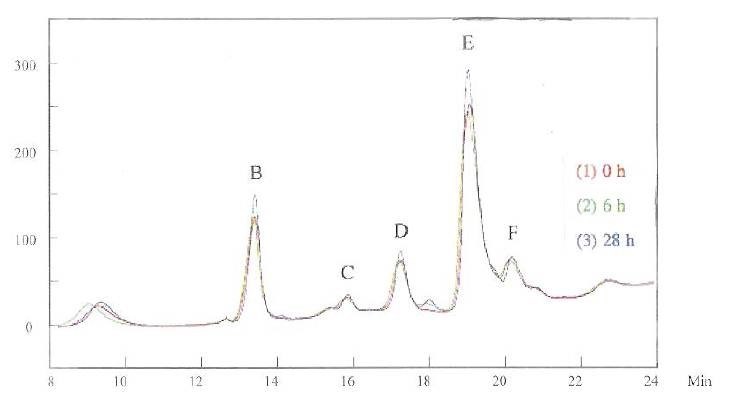
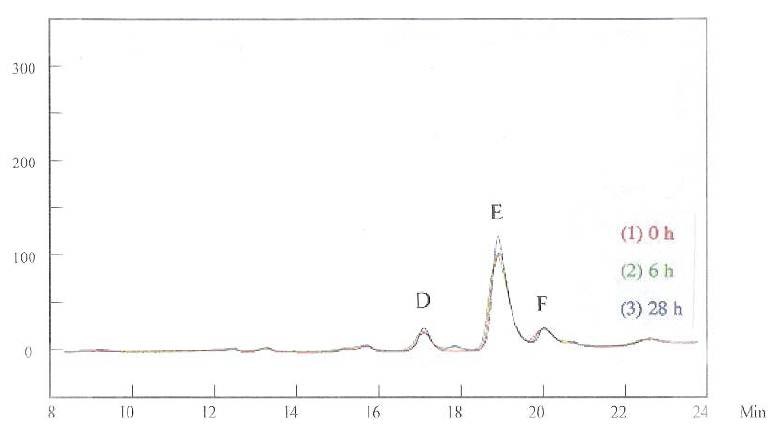
Figures 3 and 4 show the HPLC curves for the brightener in the solution, at 215 and 245 nm respectively. The first HPLC measurement was conducted immediately after the brightener was mixed with the basic copper solution, recorded as “0 h.” The measurements were then conducted six and 28 hr later, recorded as “6 h” and “28 h,” respectively. Although only one species corresponding to this measurement was expected, multiple peaks were detected at 215 nm (Fig. 3). The major peaks are marked in the figure as B, C, D, E, and F. While peaks C and F were stable, peaks B, D, and E increased after 28 hr. When the detection wavelength was changed to 245 nm, only peaks D, E, and F were apparent (Fig. 4).
Multiple HPLC peaks suggest that the brightener exists in the solution as a series of derivatives. This was confirmed by NMR and mass spectroscopic studies, which will be discussed later. These species have different sensitivities at different wavelengths, so the HPLC results are different for the measurements at 215 nm and 245 nm.
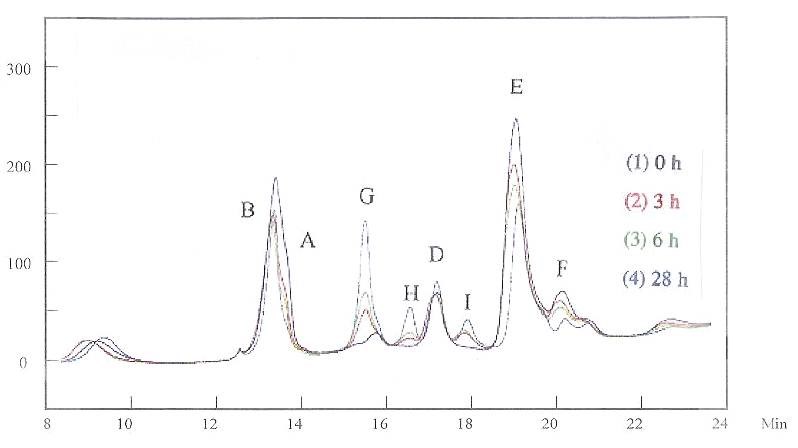
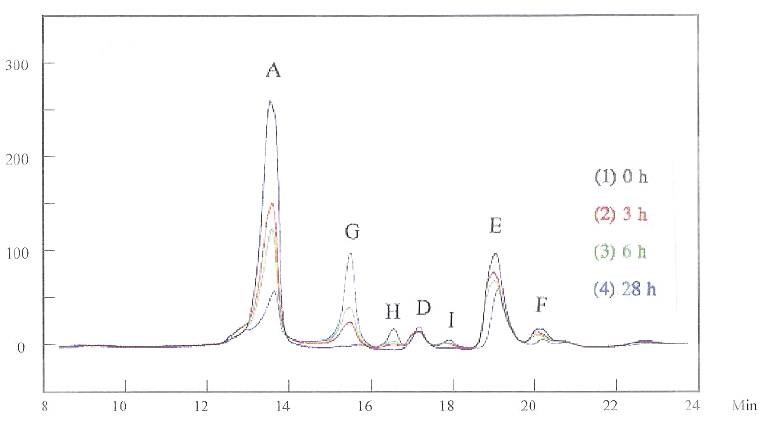
When both the grain refiner and brightener were added to the solution (Figs. 5 and 6), the HPLC peaks simply added together at the beginning, as noted in Fig. 5 for the “0 h” curve. As before, the first HPLC measurement was conducted immediately after the additives were mixed, recorded as “0 h.” The measurements were then conducted three, six and 28 hours later, recorded as “3 h,” “6 h” and “28 h,” respectively. Figure 5 was measured at 215 nm. In this figure, peaks A and B overlap somewhat. All the peaks changed with time. The original peak heights were reduced, and new peaks, G and H, appeared. The same phenomenon was noted at 245 nm (Fig. 6). Notice that no current passed for these solutions. These changes indicate that the grain refiner and the brightener have chemically reacted in the solution.
Impact of the solution reactions on the plating quality
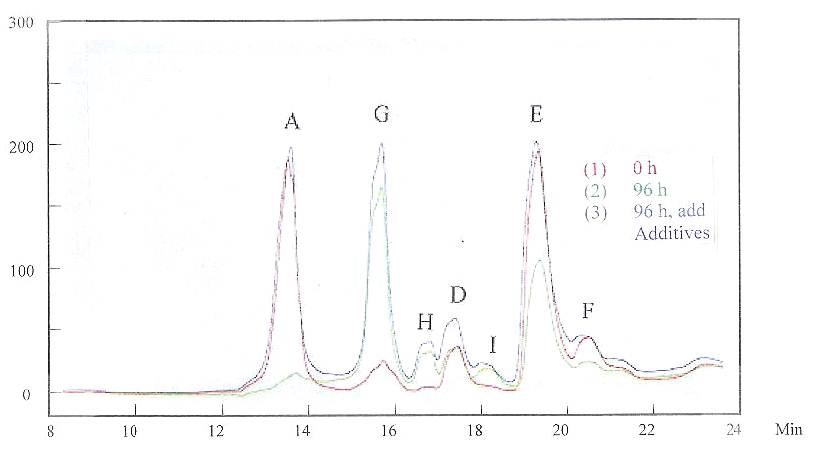
Hull Cell experiments were conducted to examine the impact of the solution change. The grain refiner and brightener were mixed in solution and then the solution was divided into six portions. The first portion of solution was used for the Hull Cell experiment immediately after the mixing, recorded as “0 h.” The Hull Cell experiments were then run 24, 48, 72 and 96 hr later in the other four portions of solution, respectively. They were marked as “24 h,” “48 h,” “72 h” and “96 h” in Fig. 7. In order to record only chemical decay and not the electrochemical reaction, no current was passed in the solutions until the Hull Cell test was conducted for all experiments. After 96 hr, both grain refiner and brightener were added into the sixth portion of solution to match the original concentrations of the additives, and the Hull Cell experiment was then conducted. The results are also shown in Fig. 7.
HPLC experiments were run in the above solutions. Figure 8 shows the HPLC results for solutions at “0 h” and “96 h,” and also after adding the grain refiner and brightener in solution. The HPLC was recorded at 245 nm. The result shows that adding additives matched the original concentrations for the species that have peaks A and E. Comparing the results in Figs. 7 and 8, it is indicated that these species are important in controlling plating quality.
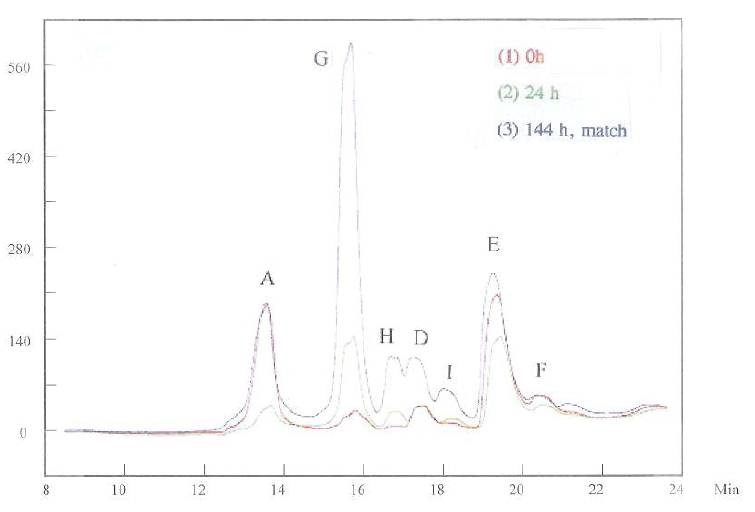
This conclusion is further supported by the results shown in Fig. 9, in which the HPLC measurements for samples “0 h,” “24 h” and “144 h” with added grain refiner and brightener were recorded. Although decomposition of additives generated huge peaks G, H and I, the Hull Cell results were similar for solution “0 h” and solution “144 h” with the additives added. It is an indication that the species with peaks A and E have much larger impacts on the plating quality compared to those with peaks G, H, and I. In other words, the consumption of additives, instead of generating byproducts, is the main reason for the unfavorable effect.
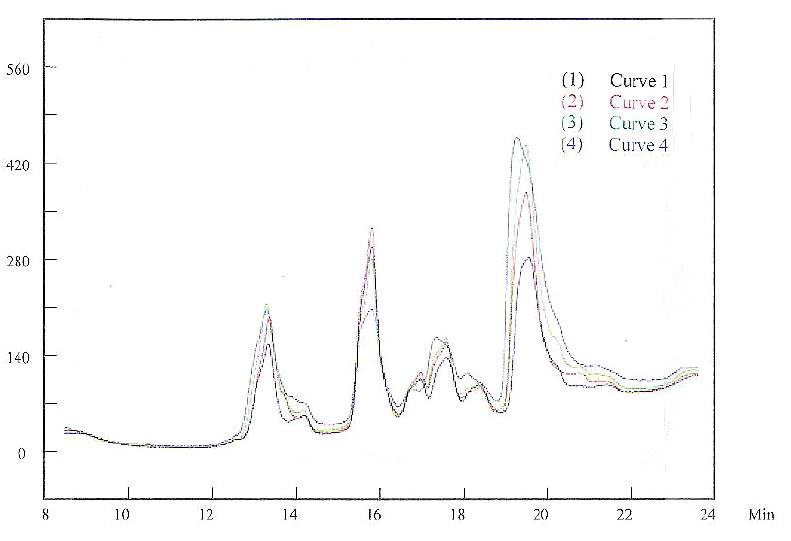
The changes that we have investigated are only chemical reactions as opposed to electrochemical ones. During electroplating, the copper plating solution was further changed. A new peak, with a retention time of about 14.1 min on HPLC, was detected. Figure 10 shows a number of HPLC detection peaks during the plating process. Because the additives were consumed in plating, they were continuously compensated for by maintaining the additive concentrations. This addition may not have exactly matched the consumption rate, which explains the different heights for the peaks in the figure. The results in Fig. 10 show that a new peak, although not well defined, appeared at about 14.1 min. Some unfavorable effects for this new species were observed when this peak appeared. For example, the copper deposited at this condition could be stressed. Therefore, the decomposition products due to the electrochemical reaction have a deleterious impact on the plating bath.
NMR spectroscopy and LC-MS were run to further understand the chemical changes in the solution associated with the grain refiner and the brightener. The results indicate that the multiple HPLC peaks for the brightener are due to its derivatives which formed in the solution. For example, the solution with the brightener is a complex mixture of mono- and di-substituted, or perhaps even tri-substituted, species. When the grain refiner and the brightener mix together, interaction occurred. Some of the functional groups in the brightener were combined with part of the grain refiner, which account for the new peaks in the HPLC measurement.
Conclusions
An acid copper solution with a grain refiner and a brightener were studied using HPLC and Hull Cell testing. The results show that the brightener existed in solution as a complex mixture of derivatives, which caused multiple peaks in the HPLC measurements. While the grain refiner itself showed relatively simple behavior in the solution, it reacted with the brightener when they were mixed together. This interaction generated new peaks in the HPLC curves. Comparing the results from the HPLC and Hull Cell studies, it was seen that the species with newly generated peaks by chemical reactions have less impact on the plating quality, while the species corresponding to the original peaks have large impacts. The decomposition products generated in the plating process have unfavorable effects for the plating bath.
Keywords: High performance liquid chromatography, acid copper plating, bath chemistry control
Read Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More
















.jpg;maxWidth=300;quality=90)






