Membrane Technology by Electroplating Processes
A broad overview of the latest developments on membrane technology and their advantages in the electroplating industry.
The efficiency of electroplating systems depends on many factors. The breakdown products are the main factor, especially in the case of zinc-nickel processes. These are generated during the electroplating process by oxidation and/or reduction of organic additives. In order to maintain a constant efficiency, membranes are installed around the anodes. These membranes are only permeable for some ions or molecules and prevent anodic oxidation reactions of the organic additives. This paper gives a broad overview of the latest developments on membrane technology and their advantages in the electroplating industry.
Introduction
Electrodeposition of zinc and nickel metal onto a ferrous substrate is a common practice for imparting protective and decorative properties to the substrate. For example, ferrous articles are often zinc or nickel electroplated to provide corrosion resistance to the substrate.
As the need for improved corrosion protection has increased over time, interest in zinc-nickel alloys has also increased, particularly as zinc-nickel coatings have been shown to provide increased corrosion protection in comparison to electroplated coatings composed of zinc alone. Alkaline zinc-nickel electrolytes are widely used in the electroplating industry. These coatings have high wear resistance, do not lose their corrosion protection when heat is applied and can be also used, when assembled with aluminum materials with regard to galvanic corrosion.1
In order to deposit a homogeneous gamma-phase zinc-nickel alloy coating, the use of amines as complexing agents is necessary. The electroplating processes using these additives tend to have poor cathode efficiency with time. During the electroplating process, breakdown products are formed by oxidation and/or reduction of organic additives. The breakdown products are the main factor reducing the performance of the zinc-nickel processes.
In order to maintain a constant efficiency, membranes are installed to separate the anodes. These membranes are only permeable for some ions or molecules and prevent anodic oxidation reactions of the organic additives. This paper will give a broad overview of the latest developments in membrane technology and their advantages in the electroplating industry.
Breakdown products
The aliphatic amines used in zinc-nickel processes have low oxidation potentials and are therefore easily oxidized at the anode. The course of oxidation depends on the structure of the amine, on the anode material and on the composition of the electrolyte.2 The mechanism is interpreted by an electron transfer from the electron pair of nitrogen to the anode. The nitriles formed produce cyanides by nucleophilic substitution with hydroxyl anions. Cyanides react with nickel from the solution to form tetracyano nickel(II) complexes. This reaction is catalyzed by nickel. Figure 1 shows the mechanism of cyanide formation.
The tetracyano complex formed is very stable and avoids further reaction of nickel. Therefore the addition of nickel and amines is necessary in order to get the proper nickel co-deposition.
These additions change the working window of the electrolyte. In particular, the current efficiency of system decreases rapidly by the addition of amines and formation of breakdown products.
A high current efficiency provides faster deposition at lower costs and the hydrogen evolution is reduced. Hydrogen evolution is always a competitive process to metal deposition. Good nucleation of the surface leads to faster overall surface coverage and reduces hydrogen evolution.
Another breakdown product formed during electrolysis is carbonate. Carbonate is also produced at the anode with intermediate formation of oxalate. Carbonate is additionally produced by reaction of sodium hydroxide with carbon dioxide from the air. Sodium carbonate concentrations higher than 60 g/L decrease the current efficiency of the system, because of the increase of viscosity.
Membranes for zinc-nickel processes
Separating the anode by a membrane prevents contact with the zinc-nickel electrolyte. The oxidation of amines and other organic compounds at the anode is thus avoided. Further, the formation of cyanide is completely prevented, which is proven by analysis during production. At the same time, the current efficiency is kept constant on a high level. The deposition of high quality zinc-nickel deposits is achieved consistently.
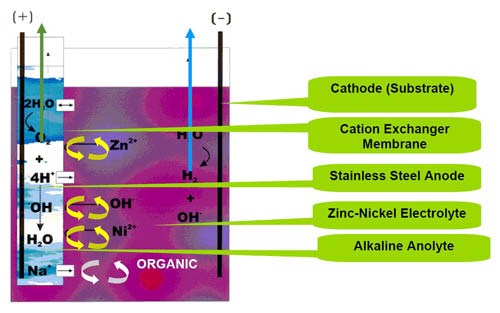
Two patented membrane types are used for zinc-nickel processes: the ion exchange membrane and the porous membrane.3,4,5 Figure 2 shows the zinc-nickel electroplating apparatus with the membrane technology applied. The apparatus comprises a zinc-nickel electroplating bath with amines and other organic additives. The bath has a pH to 14. A cathode workpiece is positioned in the catholyte compartment. An insoluble metal anode is immersed in the anolyte, which is surrounded by an ion exchange membrane. The preferred anolyte is a solution of 120 g/L sodium hydroxide and the anode material is stainless steel. A potential is applied between the anode and cathode establishing a current flow from the anode to the cathode through the ion exchange membrane. The ion exchange membrane shields the anode from the zinc-nickel electroplating bath, preventing amine breakdown into cyanides or generally speaking inhibiting the electrolytic breakdown of organic additives.
The industrially-used cation exchange membrane permits the separation of ions allowing the preferential transport of cations. If the membrane material is made from only ion-exchanging material, it is called a homogeneous ion-exchange membrane. If the ion-exchange material is embedded in an inert binder, it is called a heterogeneous ion-exchange membrane.
The cation exchange membrane is a thin sheet based on perfluorinated compounds. This membrane is very robust, especially for the high alkaline solution.
The anode can be simply stainless steel engineered in the form of a box or as tube anodes. Figure 3 illustrates the recent development in tube anode technology.
Another patented system for zinc-nickel applications is the use of an open porous membrane instead of an ion exchange membrane. The porous membrane in particular prevents the migration of large molecules, e.g., organic additives. It mainly allows the migration of ions through the membrane. The thickness and total porosity of the membrane determine the functioning of the system.
The transfer of sodium, sulfate, carbonate and hydroxide ions occurs on both sides of the membrane and the water transfer is on both sides of the membrane equivalent. Industrial measurements for water transfer (water transfer from the anolyte to the catholyte compartment) showed about 150 mL/hr for the ion-exchange membrane and 50 mL/hr for the porous membrane.3 This results in a lower volume increment of the zinc-nickel electrolyte using the porous membrane.
Comparison of a conventional zinc-nickel system with a membrane-based zinc-nickel process
The operation of the conventional zinc-nickel process has several drawbacks. Only 50 to 60% of the applied current is used for metal deposition. The rest is consumed for hydrogen and heat. Therefore the electrolyte gets warmer with time. Cyanide formation occurs during electroplating, because of oxidation of the amines. Other organic components are also oxidized and the formation of carbonate increases. Sodium carbonate at concentrations above 60 g/L must be frozen out and separated by filtration. The low current efficiency, the oxidation process and the warming of the electrolyte lead to an increased consumption of organic additives in relation to the amount of deposited metal. Finally, constant deposition parameters are achieved at a relatively low efficiency.By contrast, the membrane zinc-nickel process enjoys key benefits. Approximately 80-90% of the applied current is used for metal deposition. Lower cooling capacity is needed and the electrolyte does not warm during electroplating. Cyanide formation is avoided and waste water treatment and external disposal is eased. The membranes prevent the oxidation of organic compounds. Therefore, carbonate formation is minimized and the consumption of organic additives is reduced.
By avoiding the formation of breakdown products in the electrolyte, the nickel concentration can be kept at a constant low level and the current efficiency is maintained at a high level.
Conclusion
The technological needs of industries such as automotive, building industry, fastener and electrical components, for higher quality and longer lasting finishes with improved corrosion resistant coatings, has played a major role in the development and use of zinc-nickel coatings. Most commercial zinc-nickel electrolytes can be broadly divided into alkaline non-cyanide and acidic solutions. The alkaline non-cyanide zinc-nickel electrolyte mainly used in Europe has several drawbacks. Two main disadvantages are (1) cyanide formation due to the amine oxidation and (2) oxidation of organic additives during electroplating, which leads to a higher formation of carbonate and reduced current efficiency. These can be completely avoided by using membranes with the anodes. The two types of membranes - ion exchange membranes and porous membranes - prevent the breakdown of organic compounds at the anode and result in higher efficiency. This technology maintains the productivity and quality of the plating line at a high level.
References
- B. Sonntag, et al., Galvanotechnik, 100 (7), 1499 (2009).
- L.C. Portis, J.T. Klug & C.K. Mann, J. Org. Chem., 39 (24), 3488 (1974).
- Coventya SAS, F. Gonzalez, et al., International Patent WO 2005/073438 (2005) and corresponding international applications.
- Coventya SAS, Taskem, W.E. Eckles, et al., International Patent WO 2004/108995 (2004) and corresponding international applications.
- E.W. Hillebrand, International Patent WO 00/06807 (2000) and corresponding international applications.
_______________________
*Corresponding author:
E-mail: a.jimenez@coventya.com
Related Content
From Drain to Gain with Smart Wastewater Recovery
Incorporating digital monitoring to maximize performance.
Read MoreReplacing Open-Top Vapor Degreasing in Aerospace Manufacturing
Options and considerations for cleaning aerospace parts as regulations tighten on vapor degreasing solvents.
Read MoreHow Vapor Degreasing Helps Medical Devices Cross the Finish Line
Vapor degreaser cleaning can be simple, predictable and repeatable, making it a good fit for medical device manufacturing.
Read MoreTop Reasons to Switch to a Better Cleaning Fluid
Venesia Hurtubise from MicroCare says switching to the new modern cleaning fluids will have a positive impact on your cleaning process.
Read MoreRead Next
Masking Solutions for Medical Applications
According to Custom Fabricating and Supplies, a cleanroom is ideal for converting, die cutting, laminating, slitting, packaging and assembly of medical-grade products.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More