Metal Recovery and Wastewater Reduction Using Electrowinning
Merit Partnership pollution prevention project for finishers...
The Merit Partnership is a joint venture between the U.S. Environmental Protection Agency (EPA) Region 9, state and local regulatory agencies, private sector industries and community representatives. The partnership was created to promote pollution prevention (P2), identify P2 technology needs and accelerate P2 technology transfer within various industries in southern California. One of these industries is metal finishing, which is represented by the Metal Finishing Association of Southern California (MFASC). This project involves implementing P2 techniques and technologies at metal finishing facilities in southern California and documenting and sharing results. Technical support for this project is provided by Tetra Tech EM Inc. (formerly PRC Environmental Management, Inc.).
Electrowinning is an electrolytic technology used to recover metals from electroplating rinse waters. Although electrowinning has traditionally been used only for metal recovery, its application in a well designed and controlled rinse system can significantly reduce rinse water use, wastewater generation and chemical discharge.
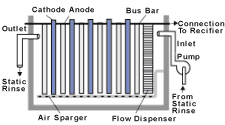
An electrowinning unit has three main components: 1) electrolytic cell; 2) rectifier; and 3) pump. An electrolytic cell is a tank in which cathodes and anodes are typically arranged in alternating order (Fig. 1). The cathodes and anodes are attached to their respective bus bars, which supply electrical potential to the unit. The electrolytic cell may include a flow disperser or air spargers to improve rinse water circulation within the cell.
When operating, the electrical potential applied to the electrodes causes dissolved metals and other positively charged ions to migrate toward and plate onto the cathodes. As metals deposit on the cathodes, the metal buildup decreases the deposition rate. When the metal deposition rate is no longer sufficient, cathodes are removed from the electrolytic cell for onsite or offsite metal recycling. In some cases, recovered metals are pure enough to be reused in process baths. As metals are chemically reduced at the cathodes, other rinse water components are oxidized at the anodes. If cyanide is present, it is oxidized to cyanate and then to carbon dioxide and nitrogen.
Technology applications. Electrowinning is most commonly used to recover gold, silver, copper, cadmium and zinc. Gold and silver are the most successfully recovered metals because of their high electropotential. Chromium is the only common electroplating metal that is not recoverable using electrowinning. Nickel recovery is possible, but the pH must be maintained within a tight range for metal deposition to occur. Some fluoroboratecontaining solutions, such as tin and tinlead solutions, can corrode certain anode materials. Most etchant solutions dissolve metal off the cathodes as quickly as they are deposited.
Design and implementation considerations. The design and implementation of an electrowinning unit depend on the configuration and control of the electroplating and rinse operations. Electrowinning can eliminate the need for continuously flowing rinse water if dragout reduction techniques and multiple rinse tanks are used.
Installing an electrowinning unit on a stagnant rinse tank after a dragout recovery tank is the most common and costeffective application of electrowinning. To maintain a steady-state metal concentration in the stagnant rinse at or below the maximum contaminant concentration, the rate of metal deposition onto the electrowinning cathodes must be greater than or equal to the rate of dragin from the preceding tank. Thus, the metal deposition rate is an essential design parameter that ultimately affects the capacity, size and cost of the electrowinning unit.
The electrowinning unit should operate 24 hours per day to maximize metal recovery and maintain the lowest possible metal and cyanide (if present) concentrations in the stagnant rinse. Operating the unit during non-production hours allows it to recover metals and destroy cyanide that accumulates during production.
Because metal and cyanide concentrations in the stagnant rinse are lowered, subsequent rinses will be cleaner, allowing the flow rates in these rinses to be reduced or turned off. In many cases, rinse water flow is reduced to a rate equal to the evaporation rate from the stagnant rinse.
Maximizing and controlling metal concentration, current density, mixing and cathode surface area will improve electrowinning performance.
Metal concentration. To achieve high recovery rates, electrowinning should be applied to concentrated rinse waters; therefore, electrowinning is most effective on a stagnant rinse.
Current density. Metal deposition occurs at faster rates with higher current densities. However, if the current density is too high, the solution surrounding the cathodes can become depleted of metals, which limits the metal deposition rate. The "excess" current applied to the electrodes is wasted on converting water into hydrogen and oxygen.
Mixing. Mixing disrupts the metal depletion layer that would form in a stagnant solution, allowing the electrowinning unit to be operated at a higher current density with a corresponding higher deposition rate.
Cathode surface area. Metal deposition rate is proportional to cathode surface area. Two main types of cathodes are flat-plate cathodes and reticulated cathodes. Flat-plate cathodes are made of stainless steel, have an effective surface area equal to their apparent area and are reusable. When deposited metal reaches a thickness of 3/16 to 1/4 inch, flat-plate cathodes should be removed and cleaned. The advantages of flat-plate cathodes are their reusability and the ability to recover metals onsite. Reticulated cathodes are made of metalcoated carbon fibers and have an effective surface area that is ten times their apparent area. The advantage of using reticulated cathodes is their high deposition rate. Reticulated cathodes are not reusable; however, fully loaded cathodes are and are sent off site for recycling.
Eventually, dissolved salts that are not removed or oxidized by the electrowinning unit accumulate in the rinse water. If these accumulated salts start negatively impacting rinsing quality, the rinse water tanks should be drained and filled with clean water. Spent rinse water can be evaporated, treated or disposed.
Case study: Electrowinning at All Metals. All Metals Processing Co. is a job shop in Burbank, CA, that performs cadmium, bronze and zinc electroplating and black oxide coating for aerospace and other industrial customers. All Metals employs 15, and its facility has about 8,000 sq ft of plating space.
In early 1996, All Metals set a goal to reduce water use and eliminate wastewater discharge to the sewer. The shop was motivated by high city sewer fees and pressure from the municipal wastewater treatment plant (POTW) to decrease the metal concentrations in treated wastewater. In cooperation with the Merit Partnership, All Metals agreed to pursue its goal in two phases. Efforts were focused on the cadmium electroplating line because it was used most frequently. Dragout from this line contributed the largest quantities of metals to the wastewater, and All Metals had exceeded its cadmium wastewater discharge limits on several occasions.
Capital. Electrowinning units typically cost $5,000 to $15,000, depending on size, design and type of cathodes used. Reusable, flat-plate cathodes cost about $200 each, and "disposable" reticulated cathodes cost about $12 each. Labor, electrode replacement, maintenance and energy costs are low.
Phase I involved evaluating overall process efficiency and control. The purpose of Phase I was to reduce dragout and optimize rinse water use before selecting or purchasing recycling/recovery equipment. Phase I modifications resulted in a 50% dragout and rinse water reduction, a 60% wastewater treatment chemical reduction, improved rinsing quality and more efficient work flow. The Phase I payback period was 1.7 years.
By improving overall process efficiency first, All Metals was able to more cost effectively apply a metal recovery technology and move toward eliminating cadmium wastewater discharges to the POTW. For Phase II, electrowinning was determined to be the most feasible technology for All Metals to reduce or eliminate wastewater discharges from the cadmium electroplating operation.
Electrowinning unit installation and operation. All Metals purchased a Retec Model 6 electrowinning unit from U.S. Filter, Warrendale, PA. The unit holds six cathodes and has a 100amp capacity. The electrolytic cell and rectifier were mounted on a shelf above a stagnant rinse tank (rinse one) that followed a spray dragout recovery tank (Fig. 2). Reticulated cathodes were used in the electrowinning unit. Anticipating that the electrowinning unit would reduce cadmium and cyanide concentrations in rinse one, All Metals turned off the rinse water flow in the subsequent counterflow rinse and converted it into a twostage stagnant rinse (rinses two and three). Rinse water was transferred from rinse two to rinse one to make up for evaporative losses. Rinse water in rinse three flows through a weir into rinse two when clean water is added to rinse three.
Ideally, a heater should be installed on the rinse tank connected to the electrowinning unit. This concentrates the influent to the electrowinning unit, increasing the metal deposition rate. It also increases the countercurrent flow of rinse water from subsequent rinses.
In addition, All Metals installed an electric heater on rinse two to increase evaporation, thereby increasing the countercurrent flow of clean water from rinse three. No heater was installed on rinse one because it was a plastic tank. All Metals also installed an in-tank filtration system on rinse two to remove suspended solids from the rinse water.
Results. The electrowinning unit operates 24 hours a day, 7 days a week. The cathodes are replaced every three months on average. Based on the start and finish weights of the cathodes, about 2 kg (4.4 lb) of cadmium were recovered from the six cathodes after two months of operation. Spent cathodes are picked up by a scrap metal dealer for recycling.
Before the electrowinning unit was installed, rinse water flowed through the counterflow rinse tank at a rate of 0.5 gpm. Since the electrowinning unit was installed, all the rinse tanks have been operated in a stagnant mode, and the temperature of the rinse water in rinse two has been maintained at about 115F, resulting in the evaporative loss of about 15 gpd. Consequently, 15 gpd of clean water per day is added to rinse three in order to compensate for the water transferred into rinse two to make up for evaporative losses. Based on comparison of water use on the cadmium electroplating line before and after installation of the electrowinning unit, rinse water use has been reduced by 94% and wastewater is no longer generated from the rinses.
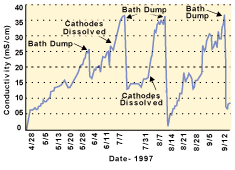
Because not all dissolved solids are removed by the electrowinning unit, conductivity is monitored in rinses one and three (Fig. 3). Rinse three is drained to dispose of dissolved solids and replenished with clean rinse water every six weeks on average. The drained rinse water is evaporated onsite. In addition, cyanide concentrations are periodically measured in rinse one. These concentrations have been significantly reduced by the electrowinning unit.
Lessons learned. Cathode installation and maintenance is critical to the electrowinning unit's performance. All Metals experienced two incidents in which the cathodes dissolved into the circulating rinse water when the electrical connection between the bus bar and cathodes was disrupted. The first incident occurred when three cathodes were improperly placed in the electrowinning unit. The second incident was caused by reuse of cathode connectors. As the connectors gradually became corroded, the electrical connection between the cathodes and bus bar was lost. All Metals now replaces the cathode connectors every time the cathodes are replaced. It also periodically checks the cathodes to assure proper connection to the bus bar.
After installing the electrowinning unit, no increase in the number of reject parts occurred, and no adverse impacts on production were observed. Motivated by the success of the electrowinning unit for the cadmium operation, All Metals has installed another electrowinning unit on a copper electroplating stagnant rinse.
The estimated 8.7-year payback period is relatively high because it considers only direct costs and savings. Other beneficial outcomes may lower the payback period. For example, the electrowinning unit takes All Metals onestep closer to its goal of zero discharge to the sewer, which will eliminate the $2,860 annual wastewater discharge fee. Also, All Metals is no longer susceptible to cadmium discharge violations, which can result in $1,000 fines.
Related Content
Headquarters Relocates for Growth and Sustainability
Gema's headquarters moves to Gossau, Switzerland, to boost production capacity and sustainability efforts with upgraded testing labs and modern infrastructure.
Read MoreHow to Maximize Nickel Plating Performance
The advantages of boric acid-free nickel plating include allowing manufacturers who utilize nickel plating to keep up the ever-changing regulatory policies and support sustainability efforts.
Read MoreTrends in EV Finishing
As OEMs put more electric vehicles — a.k.a., “green” vehicles — on the road, their colors aren’t, well, green.
Read MoreAdvantages to Pumped Eductor Agitation
Not all agitation methods are created equally. Pumped agitation with eductor nozzles can improve process tanks and quickly show a reduction in operating costs while keeping staff safe, following environmental legislation and preventing pollution.
Read MoreRead Next
Episode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More















.jpg;maxWidth=300;quality=90)










