Nanostructures by Anodization of Alumina in Phosphoric Acid at Low Potential
Anodic aluminum oxide (AAO) has been investigated, in terms of influence of the process time, anodizing potential and methanol addition on the structural features of porous anodic alumina formed in 0.1M H3PO4 solution by two-step self-organized anodizing for potentials ranging from 100-150 V. The structural features of the porous structures, including pore diameter and interpore distance, were evaluated from FE-SEM top-view images for samples anodized in the presence and absence of methanol.
by
Xavier Albort Ventura*
Laboratory Electrochemical R&D, Barcelona, Spain
University Politecnic of Catalonia, Barcelona, Spain
Tecnocrom Industrial Cabrera de Mar, Barcelona, Spain
Editor’s Note: This paper is a peer-reviewed and edited version of a presentation delivered at NASF SUR/FIN 2017 in Atlanta, Georgia on June 19, 2017. A printable version of this paper can be accessed HERE.
ABSTRACT
Anodic aluminum oxide (AAO) has been investigated, in terms of influence of the process time, anodizing potential and methanol addition on the structural features of porous anodic alumina formed in 0.1M H3PO4 solution by two-step self-organized anodizing for potentials ranging from 100-150 V. The structural features of the porous structures, including pore diameter and interpore distance, were evaluated from FE-SEM top-view images for samples anodized in the presence and absence of methanol.
For the highest anodizing time and methanol volume fraction studied, an excellent agreement between experimental values of the interpore distance and theoretical predictions was observed. The pore arrangement regularity was analyzed for various electrolyte compositions and anodizing potentials. It was found than the regularity ratio of porous alumina increased linearly with increasing anodizing potential and time. The addition of methanol improved the quality of the nanostructures. In particular, improved uniformity of pore sizes was observed in the presence of the highest methanol content studied.
Introduction
Anodization of aluminum has become one of the most popular processing methods to form porous structures with pore diameters ranging from about 10 to over 200 nm. The aluminum anodization process leading to a porous structure is usually performed in electrolytes containing sulfuric, oxalic or phosphoric acid. The structural features of the porous alumina film include pore diameters, interpore distance and porosity and they strongly depend on the chosen electrolyte and anodizing potential.
Porous alumina membranes of anodic aluminum oxide (AAO) are widely used for the fabrication of various nanostructures and nanodevices. Over the last decade many materials, including nanowires, nanotubes and nanodot arrays have been fabricated by the deposition of various metals, semiconductors, oxides and polymers inside the pores of AAO membranes (Fig. 1).
It is widely recognized that the anodization of aluminum in phosphoric acid results in nanoporous structures, but the best pore arrangement is obtained only at anodizing potentials very close to 185-205 V. Frequently, raising the potential above 170 V causes burning of the oxide layer and promotes active dissolution of aluminum instead of the desired anodic growth of porous oxide.
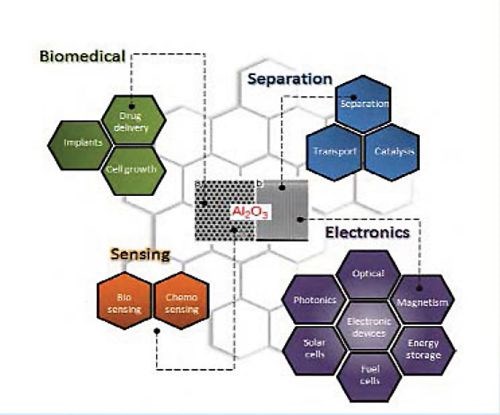
Figure 1 - Schematic diagram showing the typical AAO structure and the major applications for this nanostructured material.
Experimental process
High purity aluminum (99.999%), was used as a substrate for anodization. Prior to anodizing samples were degreased in ethanol and electropolished in a mixture of HClO2 and ethanol at constant current density of 400 mA/cm2 at 8ºC for 40 sec.
A two-step anodization procedure was employed to obtain the porous alumina structures. During the procedure, an alumina layer formed during the first anodization was chemically removed and the second anodization was then performed under same conditions used in the first step.
After the first anodization step, the oxide layer was chemically removed in a mixture of 1.5 wt% H2CrO4 and 5 wt% H3PO4 at 40ºC for 100 min.
Results and discussion
The effect of anodizing potential
The regularity of the nanopore arrangement and the structural features of porous anodic alumina were investigated for samples anodized at different cell potentials ranging from 90-150 V. Two-step anodizing of alumina was carried out in a 0.1M H3PO4 aqueous solution or a methanol-water mixture (1:3 volume ratio) at temperatures of 0 and -5°C.
A random arrangement of pores and lack of uniformity in the pore diameter and pore shape were observed for all anodizing potentials studied. This fact was qualitatively confirmed by the wide ring shapes observed in via fast Fourier transform (FFT) analysis.
In order to get a deeper insight into the regularity of pore arrangement, a regularity ratio Ri, defined as the ratio of the maximum intensity of the peak in the FFT profile to the width of the peak at half-maximum divided by the square of the interpore distance, was calculated from FFT profiles for various anodizing potentials. Determining the dependence between an average regularity ratio and the anodizing potential for anodizing carried out in water and methanol-water systems.
That interpore distance D in porous anodic alumina increased linearly with increasing anodizing potential U, according to the following equation:
D = λ U (1)
where λ = 2.5 nm/V. An average interpore distance of the nanostructure can be easily calculated from the radius of the FFT pattern. The average interpore distance calculated for all samples examined and the theoretical values predicted are shown in Fig. 2(b). A strong linear relationship between interpore distance and anodizing potential was observed to be independent of the electrolyte composition and duration of the process, as shown for samples studied in Table 1.
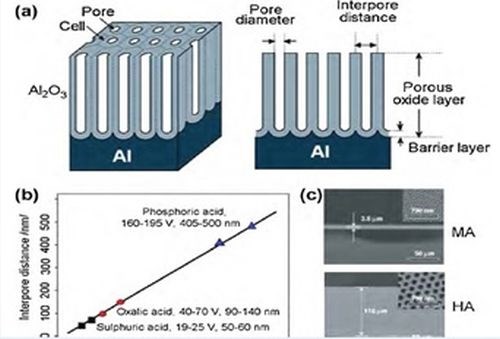
Figure 2 - (a) Schematic drawing of an anodic aluminum oxide (AAO) structure prepared by electrochemical anodization of aluminum; (b) summary of self-ordering voltage and corresponding interpore distance of AAO produced within three well-known regimes of electrolytes (sulfuric, oxalic and phosphoric); (c) (top) SEM cross-sectional view of AAO membrane formed by MA (0.3M H2C2O4, 1°C, 40 V) and (bottom) by HA (at 140 V) for 2 hr (insets: SEM top view of pore structures).
Table 1 - The proportionality constant between interpore distance and anodizing potential for various processing times of both anodizing steps.

Once again, excellent agreement between the experimental proportionality constant and the theoretical value was observed for samples anodized in a methanol-water mixture (1:4 volume ratio). This suggests that anodization of aluminum performed in a methanol-water system could lead to porous structures with a better regularity of pore arrangement.
Because porous anodic alumina films obtained by the anodizing of aluminum in phosphoric acid exhibited a complex structure with subpores formed underneath the surface (clearly visible in Fig. 3), the average pore diameter was not estimated and the analysis of the influence of potential on the pore diameter was not performed.
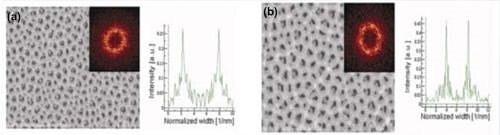
Figure 3 - SEM top-view photomicrographs with FFT images and FFT intensity profiles for samples analyzed at (a) 100 V and (b) 160 V. The anodization was carried out in 0.3M H3PO4 at 0°C. The duration of the first and second anodizing steps were both 14 hr.
The effect of anodizing time
The effect of the anodization time on the arrangement of pores and structural features of porous anodic alumina films formed in 0.1M H3PO4 at 150 V and 1ºC, was investigated in detail. Typical FE-SEM top view micrographs with FFT images and FFT intensity profiles are shown in Fig. 4 for anodizing times of 8 and 40 hr.
However, the qualitative analysis of the pore arrangement performed on the basis of the FFT images, does not give a clear picture of the effect. The quantitative inspection, exploiting the calculated average values of regularity ratio (Table 2), shows that increasing the processing time of both anodizing steps improves the regularity of pore arrangement and uniformity of pore shapes (Fig. 5).
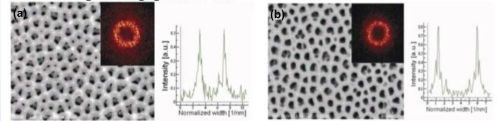
Figure 4 - FE-SEM top-view photomicrographs with FFT images and intensity profiles of FFT images for samples anodized in 0.3M H3PO4 at 160 V and 0°C. The durations of the first and second anodizing steps were (a) 8 and (b) 40 hr, respectively.
Table 2 - The influence of anodizing time on the average regularity ratio (Ri) for samples anodized in 0.3M H3PO4 at 160 V / 0°C.

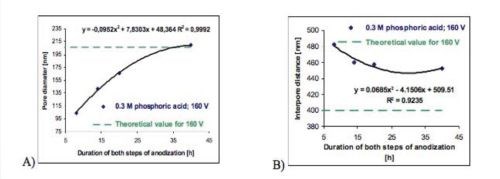
Figure 5 - The effect of the duration of both anodizing steps on (A) the average pore diameter and (B) the interpore distance of anodic porous alumina formed in 0.3M H3PO4 at 160 V and 0°C.
The effect of methanol addition
The influence of methanol addition on the regularity of the pore arrangement and structural features of porous anodic alumina was studied. The presence of methanol in the electrolyte allowed a decrease in the anodizing temperature. For the methanol addition amounts studied, the duration of both anodizing steps was 8 hr. Typical FE-SEM top-views with FFT images and intensity profiles are shown in Fig. 6 for samples anodized in a methanol-water mixture (1:4), and (1:1), containing 0.1M H3PO4.
The presence of methanol in the anodizing electrolyte influenced the structural features of porous anodic alumina. A linear decrease in the interpore distance was observed with increasing methanol content. For the highest methanol content studied, the average interpore distance observed was consistent with the predicted theoretical value from Equation 1. The effect of the methanol volume fraction on the regulatory ratio (Ri) is shown in Table 3.
Table 3 - The effect of methanol volume fraction on the regularity ratio (Ri) for samples anodized in a methanol-water mixture containing 0.3M H3PO4 at 160 V. The anodizing temperature was -5°C and the time of both anodizing steps was 8 hr.

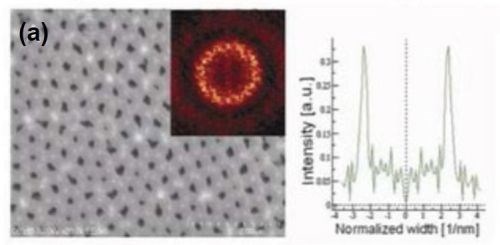
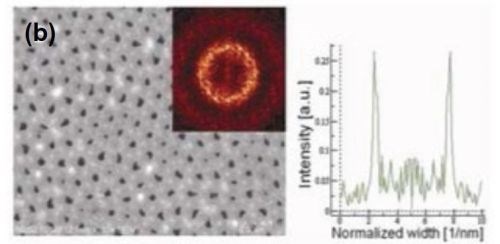
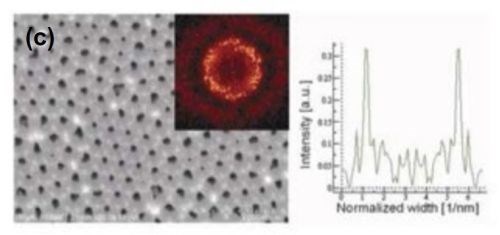
Figure 6 - FE-SEM top-view photomicrographs with FFT images and intensity profiles of FFT images for samples anodized in a methanol-water mixture (a,c)(1:4 by volume) and (b) (1:1 by volume) at (a,b) 160 V and (c) 170 V. The durations of the first and second anodizing steps were both 8 hr. The two-step anodization was carried out at -5°C.
Further studies are required to establish the optimal methanol addition for the typical temperature used for anodizing in phosphoric acid, i.e., 1ºC. Moreover, our attention is also focused on identifying the effect of different solvent additions with low boiling points on the structural features of AAO and the uniformity of the porous structure.
Summary and conclusions
Over the past several years, significant progress has been made with regard to structural engineering and surface modification of nanoporous AAO material. Much of this progress has been application-driven. In this review, we have drawn together innovative approaches on controlling and designing the structural growth of AAO with different sizes, arrangements, structures, geometries and pore architectures.
Access to these structures is achieved by changing the anodization conditions, such as current, voltage and type of electrolyte during electrochemically self-ordering of AAO. Researchers are also pushing the boundaries of molecular separations using AAO with pores of controlled shape and size, internal surface modification and studies of the effects of external parameters such as pH, flux concentration gradient and ionic strength.
Increasing anodizing potential and process time, as well as increasing the methanol content in the anodizing electrolyte considerably improve the arrangement of pores in AAO. Excellent consistency between experimental results and theoretical predictions of the interpore distance was observed for the longest anodizing time and the highest methanol content studied in the anodizing electrolyte.
About the author
Dr. Xavier Albort Ventura earned his Ph.D.in Chemistry Engineering Industrial from the University of Barcelona Spain. He is a member of the Barcelona International Trade Fair and has organizing responsibility for the Environment Technical Meetings and at the Eurosurfas show. He is an electroplating consultant for more of 50 European companies from various countries, including Portugal, France, Spain, Italy and Germany. He has been an active presenter at SUR/FIN, having given papers since 1987. He has also been an active participant at Interfinish, presenting papers and conducting workshops since 1984. He has published more than 200 papers in Spain, the United Kingdom, France, the United States and Brazil. He was awarded the Sam Wyman Memorial Award at SUR/FIN 2004 in Chicago for the Best Light Metals paper, “Sealing Method for Anodizing Aluminum and Hard Anodizing and a Test to Determine the Seal Quality of Aluminum.”
* Corresponding author
Xavier Albort Ventura, Professor and Consulting
University Politecnic of Barcelona - Spain Research and Development
C. Caravel.la La Niña, 22-24 bis
Barcelona, Barcelona Spain 08017
Phone: 34-93-2054936
Mobile: 34-63-9721065
E-mail: xavieralbort@ono.com
Related Content
How to Maximize Nickel Plating Performance
The advantages of boric acid-free nickel plating include allowing manufacturers who utilize nickel plating to keep up the ever-changing regulatory policies and support sustainability efforts.
Read MoreAn Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreTrivalent Chrome Overview
As the finishing industry begins to move away from the use of hexavalent chromium to trivalent chromium, what factors should finishers consider as they make new investments? Mark Schario, chief technology officer for Columbia Chemical offers a helpful overview of this complicated topic.
Read MorePossibilities From Electroplating 3D Printed Plastic Parts
Adding layers of nickel or copper to 3D printed polymer can impart desired properties such as electrical conductivity, EMI shielding, abrasion resistance and improved strength — approaching and even exceeding 3D printed metal, according to RePliForm.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More













.jpg;maxWidth=300;quality=90)







