NASF/AESF Foundation Research Project #120: Electrochemical Destruction of Perfluorooctanesulfonate in Electroplating Wastewaters – 7th & 8th Quarter Report
This NASF-AESF Foundation research project report covers the seventh and eighth quarters of project work (October 2021-March 2022) at the University of Illinois at Chicago. The major activities reported are: (1) to investigate 6:2 FTS oxidation, a common replacement compound for PFOS in the electroplating industry, and (2) PFAS oxidation in both a wastewater sample procured from an electroplating facility and in synthetic solutions.
By Brian Chaplin*
Department of Chemical Engineering
University of Illinois at Chicago
Chicago, Illinois, USA
Editor’s Note: This NASF-AESF Foundation research project report covers the seventh and eighth quarters of project work (October 2021-March 2022) at the University of Illinois at Chicago. A printable PDF version of this report is available by clicking HERE. A listing of previous reports to date is provided at the end of this report.
Part 1 – 7th Quarter Report (October-December 2021)
Summary
Part 2 – 8th Quarter Report (January-March 2022)
Summary
This quarter, a wastewater sample was procured from an electroplating facility, and experiments were conducted to investigate PFAS oxidation in both the wastewater sample and in synthetic solutions. Analysis of the sample determined that 6:2 FTS was the primary PFAS detected at a concentration of 220 μg/L (~0.5 μM). The ionic content was mainly comprised of NaCl and Na2SO4 salts, and the chemical oxygen demand (COD) of the sample was ~ 50 mg/L, which was attributed to other organic compounds besides PFAS.
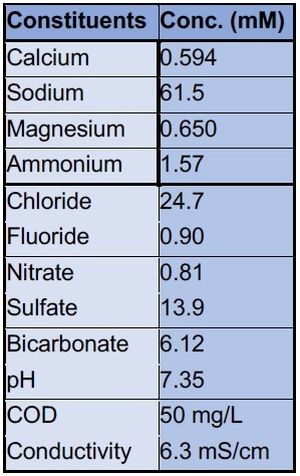
Table 1 - General water quality parameters for electroplating wastewater sample.
The wastewater sample was treated with the Ti4O7 reactive electrochemical membrane (REM), with a residence time of ~11 sec. Results for 6:2 FTS oxidation are still pending LC-MS analysis. However, the COD removal at potentials of 2.2, 2.9, and 3.6 VSHE was 10, 24, 83%, respectively. A synthetic solution with a similar ionic content to the real wastewater was prepared and spiked with 0.5 μM of 6:2 FTS. Once again, PFAS analysis is still pending, but fluoride production in permeate samples increased as a function of potential and reached as high as 4.9 μM (i.e., 75% defluorination) at a potential of 3.6 VSHE. In addition, a synthetic sample with a similar ionic strength to the wastewater was prepared but with an elevated 6:2 FTS concentration (40 μM). This sample was used to represent a PFAS concentrate from electroplating wastewater that could result from nanofiltration or foam fractionation. Results showed 66, 78, and 83% oxidation of 6:2 FTS at potentials of 2.2, 2.9, and 3.6 VSHE, respectively. Product analysis indicated the formation of short chain PFAS (i.e., PFHpA, PFHxA, PFPeA, and PFBA) at concentrations less than 1.35 μM. These results indicate that concentrating PFAS in wastewater may be a more efficient treatment strategy and that the residence time in the REM needs to be increased to prevent the accumulation of short chain PFAS byproducts.
Results
The electroplating wastewater was analyzed for PFAS and general water quality parameters. The total PFAS concentration was 228 μg/L, which consisted of 220 μg/L 6:2 FTS and low concentrations of 4:2 FTS (0.81 μg/L), PFHpA (0.38 μg/L), PFHxA (1.4 μg/L), and PFOS (2.2 μg/L). The general water quality parameters are listed in Table 1. Results indicate that the ionic content consists primarily of NaCl and Na2SO4 salts, the solution pH = 7.35, and COD = 50 mg/L.
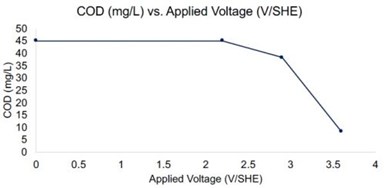
Figure 1 - Chemical oxygen demand (COD) of wastewater as a function of potential in the REM system. Residence time ~ 11 sec.
Electrochemical oxidation of the wastewater sample was tested in the REM system. The flux was held constant at 240 L/m2/hr, which corresponded to ~11 sec residence time in the reactor. Applied potentials of 2.2, 2.9, and 3.6 VSHE were tested, and PFAS removal is still pending LC-MS analysis. The COD analysis of the wastewater is shown in Figure 1, and results indicated it decreased by 10%, 24%, and 83% at potentials of 2.2, 2.9, and 3.6 VSHE, respectively. Indicating the REM was effective for bulk oxidation of the organics present in the wastewater.
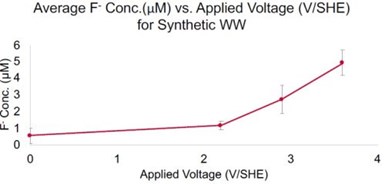
Figure 2 - Fluoride analysis for 0.5 μM 6:2 FTS oxidation in synthetic wastewater sample as a function of potential. Residence time ~ 11 sec.
An additional experiment was also conducted with a synthetic wastewater with 0.5 μM 6:2 FTS and a similar ionic content as Table 1, but without the bulk organics. The PFAS analyses is once again pending LC-MS analysis. However, fluoride analysis is shown in Figure 2. Results indicate that fluoride increases as a function of potential and reached a concentration of 4.9 mM, which corresponds to 75% defluorination of 6:2 FTS. This calculation was based on the fact that 1 mole of 6:2 FTS contains 13 moles of fluorine. Chlorate and perchlorate were also monitored, as they can form from chloride oxidation and have undesirable health risks. Perchlorate was below the detection limit (~1 μg/L) and chlorate was detected at concentrations of 1.2, 23, and 95 mg/L at potentials of 2.2, 2.9, and 3.6 VSHE, respectively. Chlorate is not currently regulated, but the EPA has set a health reference level (HRL) at 210 μg/L. Since the chlorate concentrations are much higher than the HRL, the water may need further treatment depending on discharge permitting.
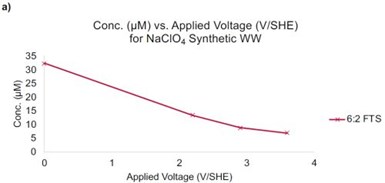
Figure 3 - (a) Concentration of 6:2 FTS as a function of the applied potential. Initial concentration of 6:2 FTS = 40 μM; (b) products from 6:2 FTS as a function of the applied potential. Residence time is ~11 sec.
An additional synthetic sample was prepared with a similar ionic strength as the real wastewater, but with an elevated 6:2 FTS concentration (i.e., 40 μM). This sample was used to represent a concentrated PFAS sample that may be generated during nanofiltration, foam fractionization, or another separation method. Oxidation experiments indicated that 66%, 78%, and 83% removal of 6:2 FTS at potentials of 2.2, 2.9, and 3.6 VSHE, respectively (Figure 3a). Product analysis indicated the formation of short chain PFAS (Figure 3b).
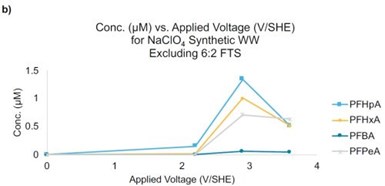
For example, the highest concentrations of PFHpA, PFHxA, PFPeA, and PFBA formed at a potential of 2.9 VSHE, with concentrations of 1.4, 1.0, 0.7, 0.06 μM, respectively. The concentrations of all of these PFAS decreased at 3.6 VSHE to concentrations less than 0.5 μM. These results indicate that concentrating PFAS in wastewater may be a viable and efficient treatment strategy and that the residence time in the REM needs to be increased to prevent the accumulation of short chain PFAS byproducts.
Previous reports
1. Quarters 1-5 (April 2019-June 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (1), 19 (October 2021); Full paper (With Project Introduction): http://short.pfonline.com/NASF21Oct1.
2. Quarter 6 (July-September 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (4), 19 (January 2022); Full paper: http://short.pfonline.com/NASF22Jan2.
About the author

Dr. Brian P. Chaplin is Associate Professor in the Department of Chemical Engineering, at the University of Illinois at Chicago. He holds a B. Civil Engineering (1999) and an M.S. (2003) in Civil Engineering from the University of Minnesota and a Ph.D. in Environmental Engineering (2007) from the University of Illinois at Urbana-Champaign.
* Dr. Brian Chaplin, Associate Professor
Dept. of Chemical Engineering
University of Illinois at Chicago
221 Chemical Engineering Building
810 S. Clinton St.
Chicago, IL 60607
Office: (312) 996-0288
Mobile: (217) 369-5529
E-mail: chaplin@uic.edu
Related Content
Masking Solutions for Medical Applications
According to Custom Fabricating and Supplies, a cleanroom is ideal for converting, die cutting, laminating, slitting, packaging and assembly of medical-grade products.
Read MoreHow to Choose Between Sulfate and Chloride-Based Trivalent Chromium
There are several factors to consider when choosing between sulfate and chloride-based baths for trivalent chromium plating. Mark Schario of Columbia Chemical discusses the differences and what platers should keep in mind when evaluating options.
Read More3 Tests to Ensure Parts are Clean Prior to Plating
Making sure that all of the pre-processing fluids are removed prior to plating is not as simple as it seems. Rich Held of Haviland Products outlines three tests that can help verify that your parts are clean.
Read MoreSurface Prep Solution for Rusted Rebar in Concrete
Julie Holmquist of Cortec Corporation discusses passivating corrosion on rebar and other reinforcing metals.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More










.jpg;maxWidth=300;quality=90)










