NASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 4th Quarterly Report
The NASF-AESF Foundation Research Board has selected a project on addressing the problem of PFAS and related chemicals in plating wastewater streams, studying PFAS destruction via electrooxidation and electrocoagulation. This fourth quarter report continues the work on electrocoagulation and electro-oxidation, measuring energy consumption and evaluating methods to remove residual zinc ions from the floc after processing.
by
Qingguo (Jack) Huang* and Yuqing Ji
College of Agricultural and Environmental Science
University of Georgia
Griffin, GA, USA
Editor’s Note: For 2021, NASF-AESF Foundation Research Board has selected a project on addressing the problem of PFAS and related chemicals in plating wastewater streams. This report covers the fourth quarter of work (October- December 2021). A printable PDF version of this report is available by clicking HERE.
Introduction
This project started in January 2021 with the goal of developing applicable electrochemical approaches to remove per- and polyfluoroalkyl substances (PFASs) present in plating wastewaters, including electrooxidation (EO) and electrocoagulation (EC). This project includes three research tasks that are designed to investigate EC, EO and EC-EO treatment train, respectively, designed to probe three hypotheses specified follows:
1) EC generates amorphous metal hydroxide flocs that can effectively adsorb PFASs in plating wastewater, which, through an appropriate treatment, can release PFASs into a concentrated solution.
2) EO enabled by a Magnéli phase Ti4O7 anode can be used to effectively destruct PFASs in plating wastewater.
3) The electrochemical treatment train comprised of EC and EO by Ti4O7 anode can remove and degrade PFASs in plating wastewater more efficiently than either process operated individually.
The results reported in the previous reports of this project demonstrated the feasibility of a novel treatment train that combines electrocoagulation (EC) with electrooxidation (EO) treatment to remove and degrade per- and polyfluoroalkyl substances (PFASs) from plating wastewater. Electrocoagulation with a zinc anode can effectively remove PFASs from water, particularly the long-chain PFASs (C7 - C10) that are present in plating wastewater, by concentrating them on the flocs or in the foams generated during EC. Both the flocs and the foams can be dissolved by acid to recover and concentrate the PFASs in controlled volumes. The concentrated PFASs in the acid solutions were efficiently destroyed using EO treatment with a Ti4O7 anode at 10 mA/cm2, and no supplemental electrolyte was needed for the flocs dissolved in solution. This electrochemical-based EC-EO treatment train can likely economically separate, concentrate and destroy PFASs in plating wastewater.
This report describes our continuing effort in Task 3. First, we calculated the energy consumption of the EO treatment process in terms of EE/O that is defined as the electrical energy required to reduce the concentration of a pollutant by one order of magnitude (kWh/m3).1 Second, we evaluated means to remove residual zinc ions that may exist after the EO treatment of the acid dissolved solution of zinc flocs.
Experimental
Calculation of EE/O was based on the results of the experiment of EO treatment reported earlier, which is presented in Fig. 2 in the 3rd report.2 In that experiment, three different concentrated solutions prepared through the electrocoagulation (EC) process were subjected to electrooxidation (EO) treatment using Magnéli phase Ti4O7 anodes at the current density of 10 mA/cm2. Solution I was the acid dissolved solution of PFASs-laden floc generated using a low current density condition after 120 min (0.3 mA/cm2, 0.005 μM each of 10 PFASs). Solution II was the acid dissolved PFASs-laden floc solution obtained through EC treatment under the high current density condition after 60 min (5 mA/cm2, 0.5 μM each of 10 PFASs). The foam collected during this EC process was supplemented with 20 mM Na2SO4 to a final volume of 10 mL as Solution III.
An experiment was performed to assess the methods of removing zinc ions from the solution produced by acid dissolution of the zinc hydroxide flocs generated during the EC process. Specifically, removal of zinc ions was achieved by precipitation with the addition of Na2S or Na2CO3. In this experiment, EC was first conducted in a 20-mM Na2SO4 solution with PFASs at 0.3 mA/cm2 for 120 min or at 5 mA/cm2 for 60 min. The entire solution, including flocs, was then collected and filtered through a 0.22-μm acetate membrane filter. The EC flocs from both current density conditions were then collected and dissolved in 10 mL 4.0M H2SO4, respectively. Na2S or Na2CO3 was then added to the solution at different dosages. The concentration of Zn2+ in the solution was determined using an ICP-MS (Perkin Elmer Elan 9000 inductively coupled plasma equipped with a mass spectrometer),3 with a detection limit of 0.05 mg/L.
Results and discussion
The EE/O (kWh/m3) of PFAS degradation in the three solutions described above was calculated by equation 1,1
(1)
where Ucell is the average cell voltage during EO treatment (V), I is the applied current (A), V is the volume of the reaction solution (L). t90% is the time (hr) for 90% PFAS removal that was calculated by equation 2:
(2)
where C/C0 is 10% and k (min-1) is the pseudo-first-order rate constant for the degradation of different PFASs in the three solutions that was obtained by fitting the data of PFAS degradation, presented in Fig. 1 of the 3rd report,2 to the pseudo-first-order rate model, which are listed in Table 1.
The calculated EE/O values of PFASs degradation in concentrated solution are shown in Table 2. The EE/O varies from 0.34 to 15.7 kWh/m3 for different PFASs in different solutions. It appears that the EE/O was lower for the long-chain PFAS, e.g., PFNA, PFOA, PFOS, than the shorter ones, e.g., PFBS and PFHxA. It is notable that the EE/O for the PFASs frequently present in plating wastewater is particularly low, for example, it was 0.66 (kWh/m3) for 6:2 FTS, 0.55 (kWh/m3) for PFOS, and 0.95 (kWh/m3) for PFOA in solution I. Such EE/O levels are considered favorable for applications in wastewater treatment.
The result of the experiment to assess the methods of removing zinc ions from the solution by precipitation with the addition of Na2S or Na2CO3 is shown in Figure 1. It is apparent that the zinc concentration remaining in the solution decreased dramatically as the added Na2S or Na2CO3 increased, because of the precipitation of ZnS or ZnCO3. Nearly all dissolved Zn2+ was precipitated out when sufficient salts had been added. This proves that chemical precipitation can be used as an effective means to remove the residual zinc in the final effluent of the proposed EC-EO treatment train.
Table 1 - The pseudo-first-order rate constant (min-1) of PFASs in concentrated solution in EO process.
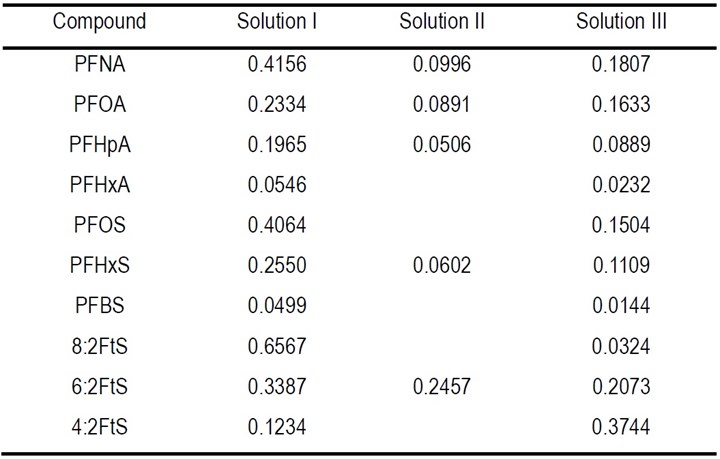
Table 2 - EE/O (kWh/m3) for PFASs degradation in concentrated solution during EO process.
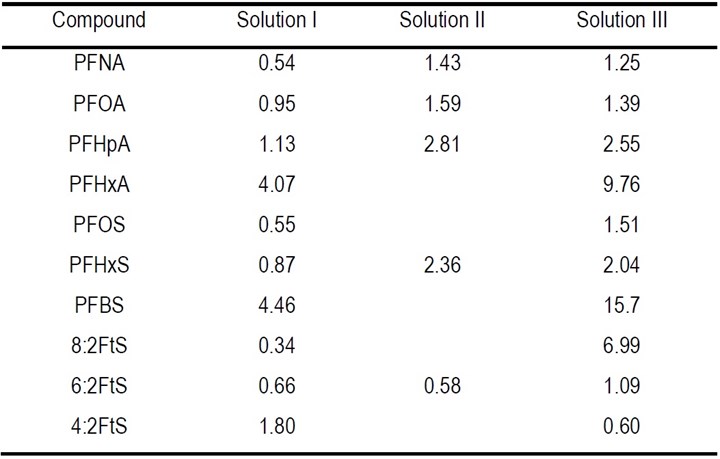
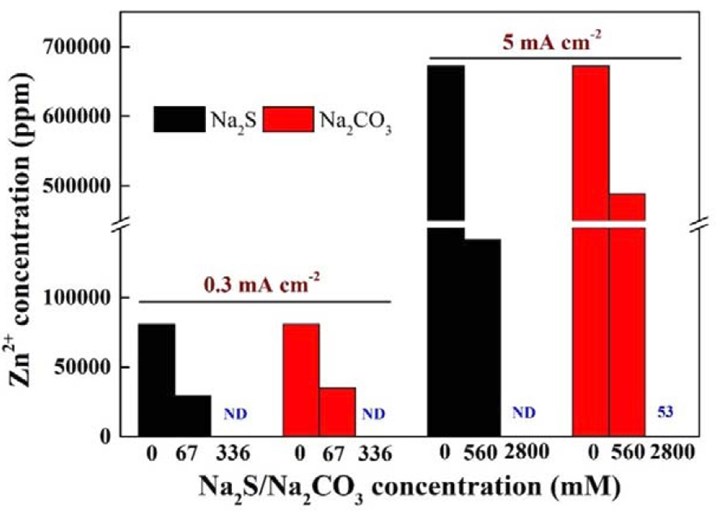
Figure 1 - Concentration of zinc ions in the solution at different current densities and with Na2S or Na2CO3 added at different dosages.
References
1. K. Yang, H. Lin, S. Liang, R. Xie, S. Lv, J. Niu, J. Chen and Y. Hu, “A reactive electrochemical filter system with an excellent penetration flux porous Ti/SnO2–Sb filter for efficient contaminant removal from water,” RSC Adv., 8 (25), 13933-13944 (2018).
2. Q. Huang, “NASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 3rd Quarterly Report,” NASF Surface Technology White Papers, 86 (6), 11-14 (2022); http://short.pfonline.com/NASF21Dec2.
3. Y. Shu, N. Zheng, A. Zheng, T. Guo, Y. Yu and J. Wang, “Intracellular zinc quantification by fluorescence imaging with a FRET System, Anal. Chem., 91 (6), 4157-4163 (2019).
Past project reports
1. Introduction to Project R-122: Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 85 (6), 13 (March 2021); Full paper: http://short.pfonline.com/NASF21Mar1.
2. Quarter 1 (January-March 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 85 (12), 13 (September 2021); Full paper: http://short.pfonline.com/NASF21Sep1.
3. Quarter 2 (April-June 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (3), 18 (December 2021); Full paper: http://short.pfonline.com/NASF21Dec2.
4. Quarter 3 (July-September 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (6), 16 (March 2022); Full paper: http://short.pfonline.com/NASF22Mar2.
About the author

Qingguo (Jack) Huang is Professor in the Department of Crop and Soil Sciences, University of Georgia, Griffin Campus. He holds a B.S. in Environmental Science (1990) and a Ph.D. in Chemistry (1995) from Nanjing University, China as well as a Ph.D. in Environmental Engineering from the University of Michigan, Ann Arbor, Michigan. Dr. Huang’s research interest focuses on catalysis involved in the environmental transformation of organic pollutants, and development of catalysis-based technology for pollution control and environmental remediation and management. His laboratory has been actively involved in several cutting-edge research topics:
Enzyme-based technology for water/wastewater treatment and soil remediation
Electrochemical and reactive electrochemical membrane processes in wastewater treatment
Catalysis in biofuel production and agro-ecosystem management
Environmental fate and destructive treatment methods of PFASs
Environmental application and implication of nanomaterials
He has published over 160 peer-reviewed journal articles, five book chapters and four patents and three patents pending. He has taught three courses at the University Georgia: Introduction to Water Quality, Environmental Measurement, and Advanced Instrumental Analysis in Environmental Studies.
* Principal Investigator (PI) Contact Information:
Qingguo Huang, Ph.D, Professor, Department of Crop and Soil Sciences
University of Georgia
1109 Experiment St.
Griffin, GA 30215, USA.
Phone: (770) 229-3302 Fax: (770) 412-4734
E-mail: qhuang@uga.edu
Related Content
NASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 10th Quarterly Report
The NASF-AESF Foundation Research Board selected a project addressing the problem of PFAS and related chemicals in plating wastewater streams. This report covers the 10th quarter of work (April-June 2023). Here, we examine the effect of surface fluorination of Ti4O7 anodes on PFAS degradation performance in terms of energy performance as well as formation of chlorate and perchlorate when chloride is present in the solution. The full paper on this work can be accessed and printed at short.pfonline.com/NASF24Feb2.
Read MoreUltrafiltration Membranes, Filter Elements for Improved Industrial Water Reuse
Ultrafiltration membranes help with water reuse in a variety of applications.
Read MoreNASF/AESF Foundation Research Project #120: Electrochemical Destruction of Perfluorooctanesulfonate in Electroplating Wastewaters - April 2022-March 2023
This NASF-AESF Foundation research project report covers project work from April 2022 to March 2023 at the University of Illinois at Chicago. The overall objective of this work is to utilize a cost-effective reactive electrochemical membrane (REM) for the removal of PFAS from synthetic electroplating wastewater. Initial results for the oxidation of PFOA with three different catalysts are discussed.
Read MoreNASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 9th Quarterly Report
The NASF-AESF Foundation Research Board selected a project addressing the problem of PFAS and related chemicals in plating wastewater streams. This report covers the ninth quarter of work (January-March 2023). In this report, we describe our work on evaluating the performance of PFAS degradation by electrooxidation using surface fluorinated Ti4O7 anodes in batch mode.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More





















