NASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 5th Quarterly Report
This paper covers a research grant at the University of Georgia - Griffin on developing electrochemical approaches to remove PFASs present in plating wastewaters, under the direction of Professor Qingguo (Jack) Huang. This fifth quarter report assessed eight PFAAs most commonly found in wastewaters, by electro-oxidation with a Ti4O7 anode across a range of anodic potentials in solutions of different compositions and at varying operating conditions.
by
Qingguo (Jack) Huang* and Yuqing Ji
College of Agricultural and Environmental Science
University of Georgia
Griffin, GA, USA
Editor’s Note: For 2021, NASF-AESF Foundation Research Board has selected a project on addressing the problem of PFAS and related chemicals in plating wastewater streams. This report covers the fifth quarter of work (January-March 2022). A printable PDF version of this report is available by clicking HERE.
Introduction
This project began in January 2021 with the goal of developing applicable electrochemical approaches to remove per- and polyfluoroalkyl substances (PFASs) present in plating wastewaters, including electrooxidation (EO) and electrocoagulation (EC). This project includes three research tasks that are designed to investigate EC, EO and EC-EO treatment train, respectively, designed to probe three hypotheses specified follows:
1) EC generates amorphous metal hydroxide flocs that can effectively adsorb PFASs in plating wastewater, which, through an appropriate treatment, can release PFASs into a concentrated solution.
2) EO enabled by a Magnéli phase Ti4O7 anode can be used to effectively destruct PFASs in plating wastewater.
3) The electrochemical treatment train comprised of EC and EO by Ti4O7 anode can remove and degrade PFASs in plating wastewater more efficiently than either process operated individually.
This report describes our continuing effort on exploring EO enabled by a Magnéli phase Ti4O7, with a focus on the perfluoroalkyl acids (PFAAs). PFAAs are particularly recalcitrant, often the end degradation products of other PFAS, and are frequently present in electroplating wastewater.1,2 PFAAs comprise some of the most well-known PFASs, such as perfluorooctanesulfonate (PFOS) and perfluorooctanoic acid (PFOA). This study assessed eight PFAAs, including perfluorobutanoic acid (PFBA), perfluopentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), PFOA, perfluorobutanesulfonic acid (PFBS), perfluorohexanesulfonic acid (PFHxS) and PFOS, by EO with a Ti4O7 anode across a range of anodic potentials in solutions of different compositions. The treatment performance was explored in terms of key operation conditions.
Experimental

Figure 1 - Photos of the electrochemical reactor: side view (left) and top view (right).
The electrochemical oxidation experiments were conducted in a rectangular reactor (10 cm × 5 cm × 2.5 cm) containing 200 mL target solution, with one Ti4O7 plate (10 cm × 5 cm) placed in the middle as the anode and two stainless steels of the same size on either side in parallel as cathodes. The reactor was made of acrylic materials, and the Ti4O7 electrodes were custom-made according to a method used in our previous study.3,4 The distance between the electrodes is 2.5 cm. The reactor setups are shown in Fig. 1.
A DC power supply unit (303 DM supplied by Electro Industries Inc) was used to supply electricity at constant current, and the solution was stirred by a magnetic stirrer at a constant speed throughout each experiment. The current density was calculated using the submerged surface area on both sides of the anode (total geometric surface area is 78 cm2). The anodic potential was monitored using a CHI 660E electrochemical workstation (CH Instruments, Inc., Austin, TX) with a Ag/AgCl reference electrode placed 0.5 cm from the anode. All potentials are reported versus the standard hydrogen electrode (SHE) with internal resistance compensation. The reaction solution was prepared with each PFAA at the initial concentration of 2.0 µM, with 100 mM Na2SO4 as supporting electrolytes, unless otherwise specified. The electric current was applied after 30 minutes of solution stirring in the reactor. Aliquots of samples, 400 µL each, were collected at prescribed time intervals, and the samples were taken after pausing the current for 90 seconds while maintaining stirring to ensure solution homogeneity. The pH was also measured during this period by an ion selective electrode (Oakton pH 300 series).

Table 1 - The flow rate and the gradient condition of the UPLC program.
Each sample was diluted 1:1 with 0.10 µM M8PFOA and M8PFOS in MeOH and filtered through a nylon-based syringe filter. Samples were processed through a UPLC-MS/MS system (Waters I-class UPLC; Water Xevo TQD triple quadrupole mass spectrometer) in a negative electrospray ionization mode using multiple reaction monitoring (MRM). The UPLC was operated with methanol (A) and water (B) (5 mM ammonium acetate in each) as the mobile phases at a flow rate of 0.3 mL/min using a gradient condition listed in Table 1.
Results and discussion
Experiments were conducted to evaluate the performance of EO with Ti4O7 anode in a solution containing a mixture of eight PFAAs mentioned above at varying current densities. Figure 2 displays the concentration profile of each PFAA in the mixture solution during electrooxidation at the current density of 5 mA/cm2 (Fig. 2A) and 15 mA/cm2 (Fig. 2B). Degradation of all 8 PFAAs was evident, with the rates greater at the higher current density. Over 90% PFOA and PFOS were removed within 30 minutes at 5 mA/cm2 and within 10 minutes at 15 mA/cm2. At 15 mA/cm2, all PFAAs were degraded over 90% except PFBA, which degraded about 50% after 8 hours of electrooxidation treatment.
.jpg;maxWidth=720)
.jpg;maxWidth=720)
Figure 2 - The concentration of each PFAA over time during electrooxidation with TSO anode at (A) 5 mA/cm2 and (B) 15 mA/cm2. The test solutions contained all PFAAs in a mixture with each at the initial solution of 2.0 μM in 100-mM Na2SO4 as supporting electrolyte.
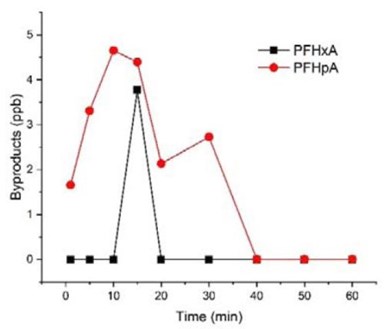
Figure 3 - Shorter chain PFAAs detected during the electrooxidation of PFOA in 100 mM Na2SO4 solution with TSO anode. Initial concentration = 2.0 μM (828 ppb for PFOA), current density = 5.0 mA/cm2.
It is possible that shorter chain PFAAs may be formed from the degradation of longer chain PFAAs. Therefore, an experiment was performed to test EO of PFOA individually in a solution. The results (Fig. 3) showed that some shorter chain PFAAs were formed as byproducts, but only account for a minimal fraction (<1%) of PFOA, consistent with our earlier findings.4 It indicated that the negatively charged substrates may be held on anode and undergo further degradation and mineralization.
The degradation of each of the eight PFAAs by EO with the Ti4O7 anode was tested with each individually in a solution at 5 mA/cm2. The data of all eight PFAAs were fitted to the pseudo-first order rate model to calculate the rate constant:
k = -ln Ct/C0×1/t (1)
where C0 is the substrate concentration at time zero (mol/L), Ct is the substrate concentration (mol/L) at time t (sec). To enable comparison between different electrodes, the surface area normalized reaction rate constant (kSA) was calculated by the following equation (2):
kSA = k × V/S (2)
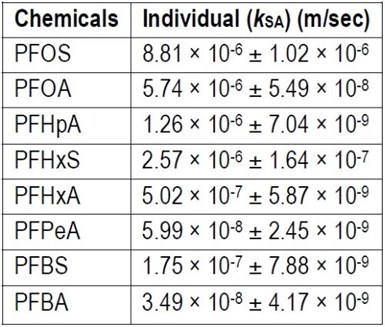
Table 2 - Surface area normalized pseudo-first order rate constants (kSA) for each PFAA by EO at 5 mA/cm2.
where V is the volume of the reaction solution (m3) and S is the effective electroactive surface area of the anode (m2). It was measured using cyclic voltammetry (CV).5 Table 2 lists the kSA values for all eight PFAAs for comparison. In general, the degradation of longer chain ones appeared to be faster, and those of perfluorosulfonic acids (PFSAs) were faster than perfluoroalkyl carboxylic acids (PFCAs) at the experimental conditions.
The impact of pH during electrooxidation was evaluated with the Ti4O7 anode at the current density of 5.0 mA/cm2 in a solution containing PFOS in 50 mM H2SO4, 100 mM NaOH or 100 mM phosphate buffer. The surface area normalized rate constant (kSA) of PFOS degradation obtained in these solutions are shown in Table 3. The kSA value was slightly smaller in the acidic solution, probably because of the lower anodic potential in this solution than that of the other two solutions. The influence of the bulk solution pH is expected to be minimal because PFOS degradation occurred on the anode surface where the pH is controlled more by the anodic reactions rather than the bulk solution.
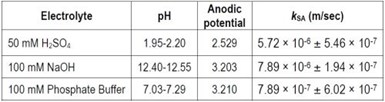
Table 3 - Surface area normalized pseudo-first order rate constant (kSA) for PFOS degradation during electrooxidation in solutions of different pH at 5 mA/cm2. Error represents standard deviation of duplicate experimental results.
A set of experiments was also performed to evaluate the electro-oxidation of PFOS with Ti4O7 in solutions containing different supporting electrolytes, including 10 mM, 25 mM and 100 mM Na2SO4, 100 mM NaNO3 and 100 mM NaClO4. The kSA of PFOS degradation obtained in different electrolytes are summarized in Table 4. At the same current density, the table shows that the anodic potential was usually higher in a lower concentration electrolyte. Therefore, kSA values decreased with increasing electrolyte concentration. The kSA did not vary much for the three different electrolyte solutions at 100 mM.
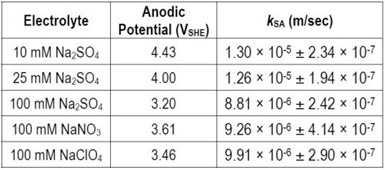
Table 4. Surface area normalized pseudo-first order rate constant for PFOS degradation during electrooxidation in solutions of different electrolytes at 5 mA/cm2. Error represents standard deviation of duplicate experimental results.
Taken together, the change in electrolyte type, concentration and pH of the reaction solution appeared to have minimal, if any, impact on PFOS degradation by electrooxidation on Ti4O7 anodes. This may indicate that the bulk solution conditions have very little effect on the anode surface conditions that are mainly controlled by the water oxidation reactions on the anode at the experimental conditions. This may be one advantage in application to waters of varying conditions.
References
- I. Ross, J. McDonough, J. Miles, P. Storch, P.T. Kochunarayanan, E. Kalve, J. Hurst, S.S. Dasgupta and J. Burdick, “A review of emerging technologies for remediation of PFASs,” Remediation, 28 (2), 101-126 (2018).
- S. Mejia-Avendaño, S.V. Duy, S. Sauve and J.X. Liu, “Generation of Perfluoroalkyl Acids from Aerobic Biotransformation of Quaternary Ammonium Polyfluoroalkyl Surfactants,” Environmental Science & Technology, 50 (18), 9923-9932 (2016).
- H. Lin, J.F. Niu, S.T. Liang, C. Wang, Y.J. Wang, F.Y. Jin, Q. Luo and Q.G. Huang, “Development of microporous Magnéli phase Ti4O7 ceramic materials: As an efficient anode for mineralization of poly- and perfluoroalkyl substances,” Chemical Engineering Journal, 354, 1058-1067 (2018).
- S. Liang, R.D. Pierce, H. Lin, S.-Y. Chiang and Q. Huang, “Electrochemical oxidation of PFOA and PFOS in concentrated waste streams,” Remediation, 28 (2), 127-134 (2018).
- H. Shi, Y. Wang, C. Li, R. Pierce, S. Gao, and Q. Huang, “Degradation of Perfluorooctanesulfonate by Reactive Electrochemical Membrane Composed of Magnéli Phase Titanium Suboxide,” Environmental Science & Technology, 53 (24), 14528-14537 (2019).
Past project reports
1. Introduction to Project R-122: Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 85 (6), 13 (March 2021); Full paper: http://short.pfonline.com/NASF21Mar1.
2. Quarter 1 (January-March 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 85 (12), 13 (September 2021); Full paper: http://short.pfonline.com/NASF21Sep1.
3. Quarter 2 (April-June 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (3), 18 (December 2021); Full paper: http://short.pfonline.com/NASF21Dec2.
4. Quarter 3 (July-September 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (6), 16 (March 2022); Full paper: http://short.pfonline.com/NASF22Mar2.
5. Quarter 4 (October-December 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (9), 5-8 (June 2022); Full paper: http://short.pfonline.com/NASF22Jun2.
About the author

Qingguo (Jack) Huang is Professor in the Department of Crop and Soil Sciences, University of Georgia, Griffin Campus. He holds a B.S. in Environmental Science (1990) and a Ph.D. in Chemistry (1995) from Nanjing University, China as well as a Ph.D. in Environmental Engineering from the University of Michigan, Ann Arbor, Michigan. Dr. Huang’s research interest focuses on catalysis involved in the environmental transformation of organic pollutants, and development of catalysis-based technology for pollution control and environmental remediation and management. His laboratory has been actively involved in several cutting-edge research topics:
- Enzyme-based technology for water/wastewater treatment and soil remediation
- Electrochemical and reactive electrochemical membrane processes in wastewater treatment
- Catalysis in biofuel production and agro-ecosystem management
- Environmental fate and destructive treatment methods of PFASs
- Environmental application and implication of nanomaterials
He has published over 160 peer-reviewed journal articles, five book chapters and four patents and three patents pending. He has taught three courses at the University Georgia: Introduction to Water Quality, Environmental Measurement, and Advanced Instrumental Analysis in Environmental Studies.
* Principal Investigator (PI) Contact Information:
Qingguo Huang, Ph.D, Professor, Department of Crop and Soil Sciences, University of Georgia
1109 Experiment St.
Griffin, GA 30215, USA.
Phone: 770-229-3302
Fax: 770-412-4734
E-mail: qhuang@uga.edu
Related Content
NASF/AESF Foundation Research Project #120: Electrochemical Destruction of Perfluorooctanesulfonate in Electroplating Wastewaters - April 2022-March 2023
This NASF-AESF Foundation research project report covers project work from April 2022 to March 2023 at the University of Illinois at Chicago. The overall objective of this work is to utilize a cost-effective reactive electrochemical membrane (REM) for the removal of PFAS from synthetic electroplating wastewater. Initial results for the oxidation of PFOA with three different catalysts are discussed.
Read MoreNovel Wastewater Treatment Targets Micropollutants
Swiss wastewater treatment technology provider Oxyle specializes in advanced wastewater treatment for removal of highly persistent micropollutants such as PFAS.
Read MoreExplore Cleaning Chemistry, Metal Finishing Applications and Wastewater Treatment Solutions
Hubbard-Hall Celebrating 175 years of excellence, Hubbard-Hall presents chemistry and equipment.
Read MoreNASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 9th Quarterly Report
The NASF-AESF Foundation Research Board selected a project addressing the problem of PFAS and related chemicals in plating wastewater streams. This report covers the ninth quarter of work (January-March 2023). In this report, we describe our work on evaluating the performance of PFAS degradation by electrooxidation using surface fluorinated Ti4O7 anodes in batch mode.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More








.jpg;maxWidth=300;quality=90)












