
RI EN
Photo Credit: MacDermid Enthone Industrial Solutions
Legislative directives have long been one of the primary drivers for innovation in surface finishing. As environmental considerations continue to be at the forefront of discourse, demands from law-making authorities continue to push researchers to meet new regulations.
Nickel continues to be a substance of interest to health officials worldwide as nickel compounds are classified as human carcinogens(1)(2)(3)(4). The Occupational Safety and Health Administration (OSHA) has established workplace exposure limits for those who work with nickel and its compounds for the associated health concerns.
Electroless nickel chemistries are typically operated at elevated temperatures and generate emissions at a higher rate than ambient temperature processes. For these reasons, reduced ion electroless nickel technology (RI EN), or electroless nickel that operates at significantly lower metal concentrations than conventional chemistries, has an important role in the future of sustainable, ecologically minded EN processes. Throughout the development and subsequent qualification of this type of chemistry, some fundamental performance advantages were discovered and characterized. These are the primary focus of this paper. Three main advantages of RI EN compared to conventional EN processes will be discussed.
- RI EN provides improved tolerance for orthophosphite by-product formation
- RI EN provides a coating that has an intrinsic stress that is more compressive
- RI EN operating solutions have a lower amount of total dissolved solids
Brief description
Electroless nickel (EN) deposits are a class of coatings defined as “functional” which are used primarily to enhance the surface performance properties of variousconductive and non-conductive substrates. The majority of applications require that the coating provide maximum protection against corrosion and abrasive wear, resulting in an extension in the useful life of the component(9). RI EN has demonstrated an effect on several of the fundamental mechanisms which serve to improve the overall performance of both the operation of the plating solution and the EN film itself.
Orthophosphite tolerance
In simplified terms, conventional electroless nickel-phosphorus deposition occurs as the result of a continuous catalytic reaction where a phosphorus containing reducing agent (typically sodium hypophosphite) is oxidized at a catalyst. As a result of this oxidation, electrons are liberated and these electrons then reduce nickel and phosphorus ions at the catalyst to form the coating itself. In the case of electroless nickel, the Ni film is the catalyst, so the deposition reaction is characterized as autocatalytic in nature. The formation of by-products, specifically orthophosphite (oxidation product of hypophosphite), during the plating process prevents the EN plating bath from being used for extended periods. In practice, the useful life of an EN plating process is described in terms of Metal Turnovers (MTOs), where one MTO is equivalent to the replenishment of the initial nickel concentration. In other words, if the original concentration of nickel ions in solution is 6.0 grams per liter (g/L) and subsequently 6.0 g/L of nickel has been deposited out of that solution, the bath is defined as “at 1.0 MTO” and so on in direct proportion to the amount of nickel ions deposited and replenished. In most conventional EN systems, the average orthophosphite generation for each MTO achieved is approximately 23 grams per liter and consequently, the plating solution becomes highly concentrated with orthophosphite anions as the EN solution is used. As previously stated, this ultimately leads to the finite life of an EN solution due to eventual solubility and performance related effects. At sufficient concentrations and conditions, free nickel ions can combine with the orthophosphite anion to form an insoluble precipitate as shown below.
Ni2+ + 2H2PO3- → Ni(H2PO3)2 ppt
These insoluble species can interfere with the formation of the EN layer and cause heterogeneities in the EN layer and can lead to increases in tensile stress and higher corrosion of the deposit in certain environments. To prevent this detrimental condition from occurring, chelation is added to EN solutions. Chelates in EN formulations are usually organic molecules that have moieties that can donate electron density to the electropositive nickel ions and thus form complexes. The relative strength of these complexes and the concentration of the chelator(s) must be enough to prevent the formation of nickel orthophosphite to be considered effective. Examples of common chelators in electroless nickel formulations are carboxylic acids such as citric acid and malic acid. Theoretical calculations can be made, based on the number of donor groups of the chelator, the molar mass and the concentration of the chelator and the concentration of Ni ions in solution, which determine how much of the Ni ions are theoretically complexed. This can be qualified as the percentage (%) of Ni ions that are sufficiently complexed in the EN bath. So at 100% chelation, theoretically 100% of the Ni ions are sufficiently complexed. This, of course, is an oversimplification of what is physically occurring in an EN solution as many other considerations must be taken to actually characterize the state of complexation in the bath but is used for the purposes of this paper as a comparative tool for illustration of the effect of the RI EN. Figure 2 demonstrates the effect of solubility of the Ni ions in an RI EN bath at 3.0 g/L Ni ions and a conventional EN solution at 6.0 g/L Ni ions in the presence of a constant concentration of orthophosphite (60 g/L) and two Ni ion chelation % values at typical working temperature of bath (i.e. 190°F). To demonstrate this effect, an alkaline (i.e., dilute NH4OH) was titrated into the solution until observable nickel orthophosphite precipitate was formed. The chelation systems in each bath were identical in composition and ratio of concentration of each complexor to the concentration of Ni ions but varied in absolute chelator concentration to effectively yield the stated chelation %. Figure 1 represents the formulations utilized for all testing in this paper. Note that succinic acid functions as a buffer in this formulation due to steric hindrances that result because of the length of the carbon chain of the dicarboxylic acid. As a consequence, no chelate ring can form with a Ni ion, thus it cannot act as a chelator.
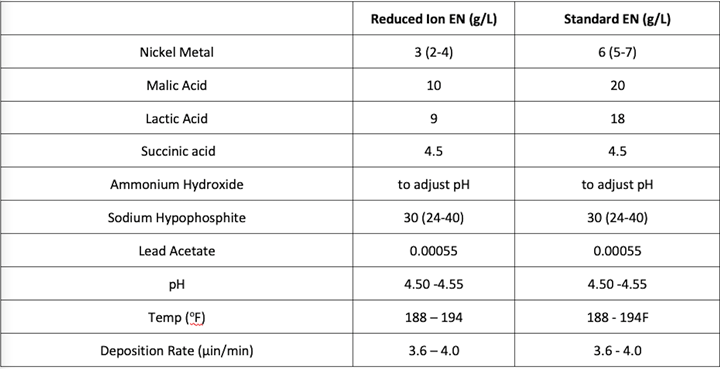
Figure 1. EN formulations
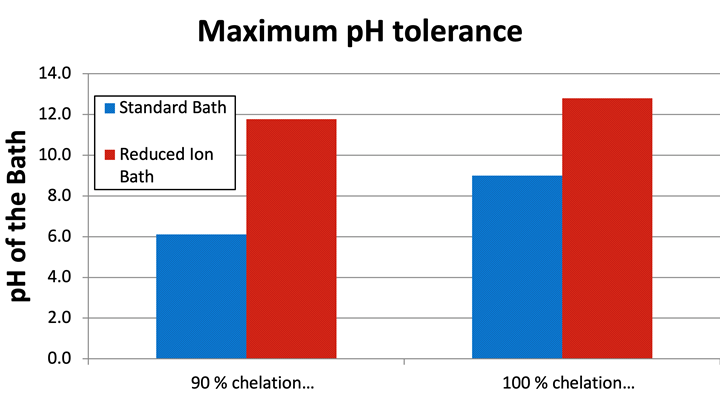
Figure 2. Nickel orthophosphite formation in RI EN and conventional EN at 90% and 100% effective Ni ion chelation at T = 190°F
Figure 3 shows essentially the same effect. This test was conducted at two different orthophosphite concentrations (i.e. 60 and 90 g/L) and the two baths were at only 55% theoretical Ni ion chelation. The pH of the solution was adjusted with alkali (i.e., dilute NH4OH) to the stated values in the figure and held constant. The temperature of the EN bath was then increased at a rate of 2°F/min and the temperature at which a precipitate formed was recorded and plotted in the figure below.
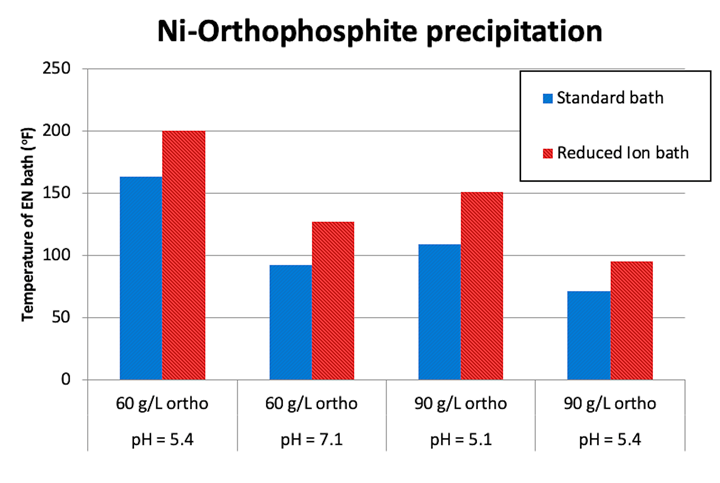
Figure 3. Effect of orthophosphite concentration and pH on the temperature at which nickel orthophosphite forms in RI EN and Conventional EN
According to Figures 2 and 3, the RI EN bath shows insolubility of the Ni ions at higher pH values as compared to the standard bath. This shows that the RI EN has a higher tolerance for orthophosphite which, based on previously stated findings, improves the performance of the coating by preventing the formation of the insoluble particles at the plating surface, which reduces the potential for both co-deposition of these particles or their potential for interference with the growth of the EN film.
Intrinsic stress
Electroless nickel deposits contain internal stresses that are the result of two individual components, extrinsic and intrinsic stress. Extrinsic stress is caused by the thermal expansion coefficient differences that exist between the coating and the substrate(6). Intrinsic stress results from crystal and lattice growth defects introduced into the coating during deposition(7). Generally, higher tensile stress values lead to microstructural mismatches in the deposit which can result in heterogeneities in the deposited layer which can lead to reduced corrosion resistance of the deposit. It has been demonstrated that deposits with compressive stress exhibit enhanced resistance to chemical attack(7). In general, EN deposits that have lower (or more compressive) intrinsic stress are more desirable.
Intrinsic stress was measured by the bent strip method, where a very thin test strip is plated in the solution of interest. The strip is split into two legs with opposing sides coated with a lacquer to prevent plating so that when the EN layer is deposited onto the non-lacquered or plateable side of the strip to a sufficient thickness, the intrinsic stress of the deposit causes the strip to deform either as a concave or convex arc. The shape of the deformation is determined by the relative stress type (e.g., compressive or tensile), the degree of stress in the deposit and the thickness of the deposited layer. The strip is then mounted on a measurement device and based on the degree of deformation, the strips separate from one another and the value is derived from the measurement scale. This value is then used as part of a calculation and the intrinsic stress of the deposit is determined in pounds per square inch (psi). This methodology was developed by Specialty Testing and Development Co., Seven Valleys, PA, and is widely used in several metal finishing chemistries to determine deposit stress.
Figure 4 illustrates the finding that the deposit generated from the RI EN bath demonstrates lower (or more compressive) stress values when tested at varying conditions. The baths contained chelation systems which were identical in composition and ratio of concentration of each complexor to the concentration of Ni ions, but varied in absolute chelator concentration to effectively yield the equivalent chelation %. Note that at the 4 MTO, the standard or conventional EN bath exhibits a deposit with tensile stress, whereas the RI EN deposit still retains compressive intrinsic stress. Figure 5 illustrates this effect again by testing stress of the deposits generated from the two aforementioned baths at 4 MTO at two different pH values. Again, the RI EN deposit yields a consistently lower compressive stress value as compared to the conventional EN bath. It should also be noted that the figure illustrates that at higher pH, the stress increases in the tensile direction.
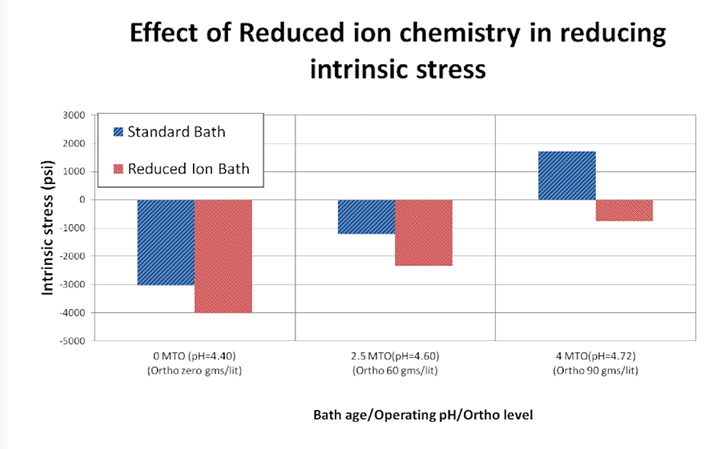
Figure 4. Relative stress of RI EN and conventional EN deposits at varying conditions
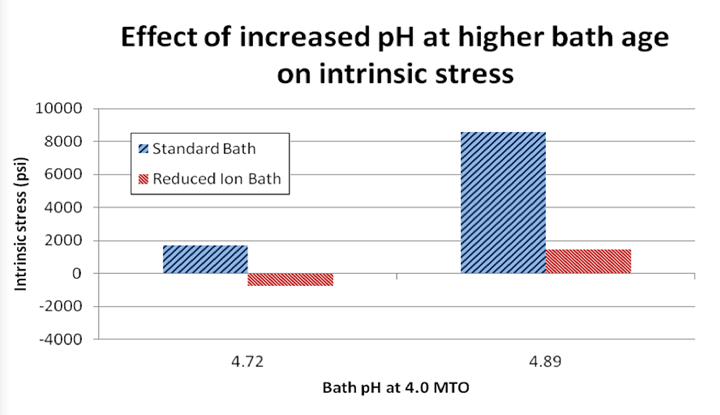
Figure 5. Effect of pH on intrinsic stress of EN deposit at 4.0 MTO, T = 190°F
According to Figures 4 and 5, the RI EN bath, when plated at comparable operating conditions, as compared to a conventional EN formulation of the same composition, generates deposits that exhibit lower intrinsic stress. This will lead to improved resistance to corrosive environments. This effect is demonstrated by Figure 6 where the two aforementioned formulations from Figure 1 were used to generate deposits of a thickness of approximately 400 µin on aluminum substrates. The pretreatment process prior to EN consisted of a non-etch soak/acid etch and a “standard” double zincate process. This process has been shown to yield very good adhesion of the EN layer to the aluminum substrate. Once plated, the thickness of the EN deposit was verified via x-ray fluorescence. These specimens were then immersed into a solution 50% v/v deionized H2O and 50% v/v 42° Baume nitric acid at a temperature of 72-74°F for 25 minutes. The weight loss was determined by taking the mass of the cleaned and dried specimen prior to and post immersion in the etch solution. The resultant weight loss was then used to derive an etch rate or corrosion rate by dividing this mass loss by the total area and the immersion time to yield a value represented by the unit (mg/cm2/min). The relative corrosion performance was characterized by comparing the data set to the highest value obtained in the experimental matrix and calculating the relative corrosion rate as a % of that value.
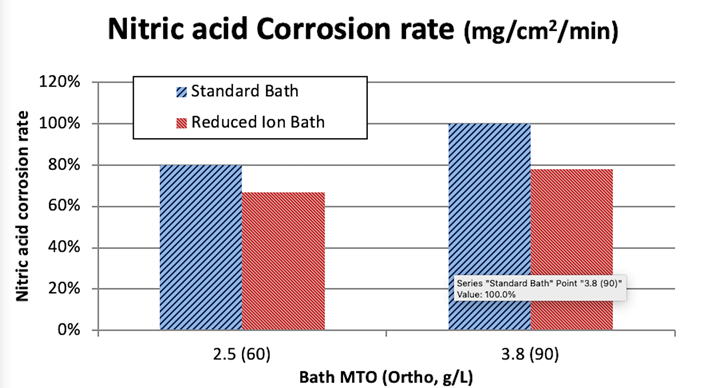
Figure 6. Corrosion comparison of deposits generated from RI EN and conventional EN in nitric acid.
Based on Figure 6, it can be stated that the RI EN deposit exhibits higher corrosion resistance as compared to the deposit generated from the standard EN deposit.
Solution density
Density is a measure of a substance’s mass per unit volume. Traditionally, solution densities have been characterized as grams per cubic centimeter (g/cm3). The reduction of Ni ions in solution by 50% allows for the reduction of other species in the electroless nickel formulation without the sacrifice of performance and, as a consequence, the density of the solution at the same MTO is reduced. This condition may impact the reduction of particle adsorption and the subsequent encapsulation of particles during the plating operation by reducing the number of dissolved ions both in the bulk of the solution and at the plating surface, thus allowing for improved migration of these particles away from the film. These undesirable particles may be generated in situ in the electroless nickel plating bath or can be introduced from the external environment. Figure 7 illustrates the reduction in the solution density as a function of the MTO of the bath.
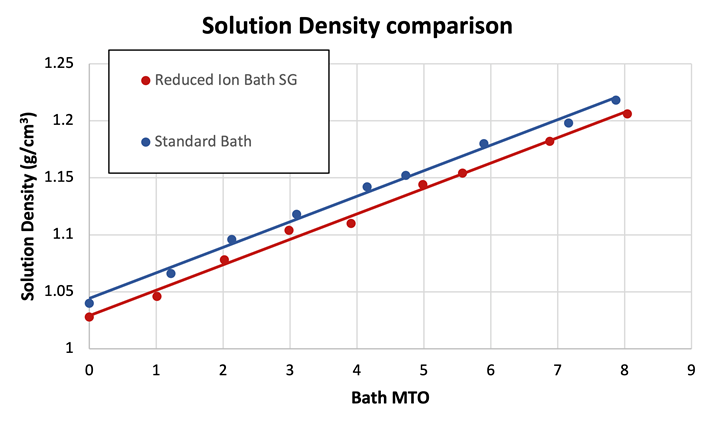
Figure 7. Solution Density of RI EN bath and standard bath over the operating life
Due to the previously stated mechanistic implications of operation of an EN solution at reduced solution density, it can be theorized that the degree of co-deposition of undesirable particles will be reduced and thus a more homogenous EN film will result. This will ultimately improve the performance of the film and could be a contributing factor to the enhanced performance demonstrated by the corrosion test previously shared (Figure 6).
Conclusion
Movement toward sustainability in the metal finishing industry, whether imposed by outside forces or as a result of internal motivation, can be viewed as an impediment to progress by some, but these challenges can also present opportunities for innovation and improvement to our industry and to the technologies that our industry employs. The creation of reduced ion ENtechnology is an example of this opportunity. This paper demonstrates the fundamental improvement of the reduced ion technology compared to current technologies.
About the Author

Ambrose Schaffer
Ambrose Schaffer is the electroless nickel global product line manager for MacDermid Enthone Industrial Solutions.
References:
(1) International Agency for Research on Cancer (2012). “Nickel and nickel compounds” in IARC Monographs on the Evaluations of Carcinogenic Risks to Humans. Volume 100C. pp. 169–218.
(2) Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labeling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC) No 1907/2006 [OJ L 353, 31.12.2008, p. 1]. Annex VI.
(3) Globally Harmonised System of Classification and Labelling of Chemicals (GHS), 5th ed., United Nations, New York and Geneva, 2013.
(4) National Toxicology Program. (2016). “Report on Carcinogens”, 14th ed. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service
(5) Nickel Risk Assessment, retrieved from https://www.nickelinstitute.org/en/Sustainability/NickelRiskAssessment.aspx
(6) Mallory, G.O. and Hajdu, J. B., (1990) Electroless Plating: Fundamentals and Applications pp. 1 – 99
(7) Mallory, G. O. and Altura, D., “The Effect of Stress on the Properties of Electroless Nickel — Phosphorus Deposits” SAE Transactions Vol. 92, Section 3: 830693–831395 (1983), pp. 1-9
(8) Ruffini, A. J. et al, (2018). US2018209047 (A1) Washington, DC: U.S. Patent and Trademark Office.
(9) LaPlante, J. (2005) “Building a platform for growth: Technology advances in electroless nickel provide practical benefits for platers” Metal Finishing Vol. 103, Issue 12 pp. 18-24
Read Next
An Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreEpisode 23: An Interview With Brad Durkin, technical director for the Electroless Nickel Conference 2022
Electroless Nickel Conference 2022 will take place October 11-13 at the Omni Severin in Indianapolis, Indiana. Learn more about the event in this interview with ENC22 technical director Brad Durkin.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More


















