Removing Heavy Metals from Wastewater
The removal of soluble heavy metal ions from wastewater is a common industrial treatment requirement in modern manufacturing.
Q: What are some tips you can give our shop about the proper removal of soluble heavy metal ions from from our wastewater?
A: Today’s manufacturers should be keenly aware of the problems—and especially the penalties—associated with discharging industrial wastewaters into sewage treatment systems. Corrosion and other interference with those systems, exposing workers to toxic substances and hazardous fumes, expensive sludge disposal costs and the pass-through of toxic pollutants into surface waters are just some of the results of improper or insufficient wastewater treatment.
The removal of soluble heavy metal ions from wastewater is a common industrial treatment requirement in modern manufacturing. The processes that generate waste streams containing heavy metals include:
- Electroplating
- Electroless nickel plating
- Printed circuit board manufacturing
- Metalforming operations
- Battery recycling
- Mining operations
Precipitation and ultrafiltration provide two ways to remove heavy metals from wastewater that are superior to conventional solutions. Both can help manufacturers avoid costly consequences, and a reputable provider can advise on which process will deliver the best results based on a variety of application-specific criteria.
Understanding the Variables
The concentration and chemical form of the soluble heavy metals in a particular wastewater stream vary, depending on the industry and the mix of operations at a processing site. For example, in the plating industry, specialty chemical suppliers have developed alloy technologies such as zinc/nickel and other alloy coatings to maximize the high corrosion resistance of the metal.
The zinc/nickel of choice for today’s surface finishing industry is an alkaline electrolyte chemistry instead of an acid electrolyte chemistry. This is due to the alloy distribution required by the plate thickness throughout the entire part. Unfortunately, alkaline chemistry contains chelators and/or complexors and organic additives to allow for the co-deposition of the nickel in the deposit with the zinc. These chelators/complexors make the wastewater extremely difficult to treat through conventional chemical or physical treatment methods.
Zinc and chrome wastewater can be treated through conventional methods, such as precipitation and the use of polymers in a lamella clarifier, followed by a filter press to dewater the sludge. The highly complexed alkaline zinc/nickel, however, doesn’t want to precipitate and the chelated metals tend to stay in solution. Additionally, the metal concentrations are too high to discharge to the local publicly owned treatment works (POTW). Nickel is a hazardous constituent and is not exempted. The addition of wastewaters from the zinc/nickel process will cause your filter press cake to become a “listed” RCRA hazardous waste with the F006 designation, resulting in significant increases to your disposal and regulatory costs.
Removing the Solids
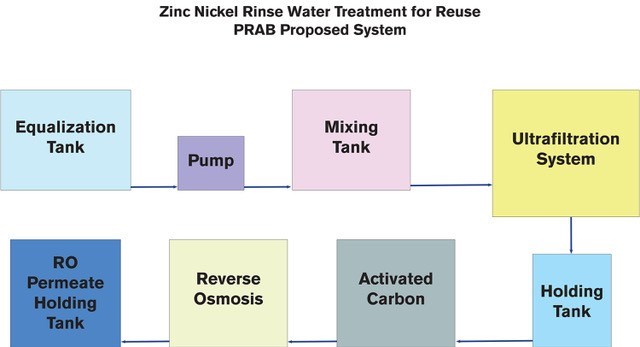
Typical removal strategies involve precipitating the metals in an insoluble form—such as hydroxides, sulfides, carbonates or some combination—and removing the precipitate with Tubular ultrafiltration (for very high-quality filtrate) or conventional clarification. The resultant sludge is then collected, thickened and dewatered for landfill disposal and listed as F006 (but at a much smaller volume) and the water phase is returned to the ultrafiltration process.
Unlike conventional clarifier techniques, tubular ultrafiltration (UF) is a very effective method of removing virtually all of the precipitated metal hydroxide/sulfides/carbonates from the treated wastewater stream. The resulting high-quality permeate can be fed directly to reverse osmosis equipment for reuse, reclaimed “as is” or discharged to the POTW. Tubular ultrafiltration can process solids concentrations of up to 18 percent (w/w), but the most efficient operation normally occurs between 3 percent and 5 percent solids.
The sludge is normally drawn off and passed through conventional thickening/filter press processes or similar processes to produce dewatered solids, typically for landfill disposal. Supernatants from the sludge handling processes are recycled through the TMF system so that the only outputs are the filtered water and the compact solids cake.
Hydroxide precipitation is a common method as it is relatively simple to operate. Sulfide precipitation has some advantages, but pH and oxidation-reduction potential must be carefully controlled to minimize the risk of producing toxic levels of hydrogen sulfide gas. Using hydroxide and sulfide precipitation in two sequential steps is also an option, particularly where complexes or chelates are present. Carbonate coprecipitation using sodium or calcium carbonate can also be helpful; for example, for soluble lead reduction, lead carbonate is essentially insoluble (0.00011 g/100 mL at 20°C) and will precipitate out. Phosphate precipitation is another option, although this process is not as common.
Finally, there are membrane technologies available that utilize a proprietary additive in conjunction with a chelate breaker for precipitating the chelated metals. The advantages of this process include:
- Eliminating the need for clarifiers or lamella separators.
- Not requiring coagulants for processing because the pore size of the microfiltration and ultrafiltration membrane is so small.
- Reducing the volume of sludge generated.
- Lowering labor costs by reducing the need for operator interface and constant monitoring.
- Lowering ch0emical costs.
- Producing a more consistent effluent.
Wastewater treatment can understandably appear to be a daunting task, especially when heavy metals are part of the equation. Partnering with a proven expert in wastewater solutions for a variety of industries, chemistries and applications can ease your burden and improve your profitability as you address an important and essential aspect of modern manufacturing.
Tim Hanna is the vice president of business development for PRAB. For information, visit prab.com.
Related Content
Innovation in Plating on Plastic
Plating on advanced plastics solution offers improved adhesion, temperature resistance and cost savings.
Read More3 Tests to Ensure Parts are Clean Prior to Plating
Making sure that all of the pre-processing fluids are removed prior to plating is not as simple as it seems. Rich Held of Haviland Products outlines three tests that can help verify that your parts are clean.
Read MoreHow to Maximize Nickel Plating Performance
The advantages of boric acid-free nickel plating include allowing manufacturers who utilize nickel plating to keep up the ever-changing regulatory policies and support sustainability efforts.
Read MoreHow to Choose Between Sulfate and Chloride-Based Trivalent Chromium
There are several factors to consider when choosing between sulfate and chloride-based baths for trivalent chromium plating. Mark Schario of Columbia Chemical discusses the differences and what platers should keep in mind when evaluating options.
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More

















.jpg;maxWidth=300;quality=90)








