Special Aspects of Electrodeposition on Zinc Die Castings
Electroplating is a frequent choice to give zinc die cast components a high-quality protective and decorative surface finish, however, applying this kind of treatment for die cast zinc components presents hidden challenges. In order to overcome these issues, a thorough morphology and composition analysis of die cast items was conducted. Special aspects of zinc die cast as a plating substrate are described and linked to the die casting process.
by
Valeriia Reveko1,2* and Per Møller2*
1Collini GmbH, Hohenems, Austria
2Technical University of Denmark, Lyngby, Denmark
Editor’s Note: A printable version of this paper can be accessed HERE.
ABSTRACT
Metal die casting has become a popular choice for the manufacturing of different consumer goods. Among the various engineering alloys and other metals, zinc alloys have often been preferred by manufacturers due to their qualities such as castability, dimensional stability and moderate solidification temperature, which provides energy and cost savings. Electroplating is a frequent choice to give zinc die cast components a high quality protective and decorative surface finish. However, applying this kind of treatment for die cast zinc components presents hidden challenges. In order to overcome these issues, a thorough morphology and composition analysis of die cast items was conducted. Special aspects of zinc die cast as a plating substrate are described and linked to the die casting process.
Keywords: electrodeposition, zinc die cast, surface finishing, electroplating defects
Introduction: why do we need to improve zinc die cast parts?
Currently, metal die casting has become a popular choice for the manufacturing of finished products. An increasing number of industrial producers are attracted by this highly economical and efficient process capable of providing near-net shaped components that fully satisfy the geometrical requirements.
Zinc, as a basis material, offers a broad range of desirable properties, beginning with hardness, ductility, impact strength, self-lubricating characteristics,1 and exhibits excellent thermal and heat conductivity.
Among the various engineering alloys and other metals, zinc alloys have often been preferred by manufacturers because of their castability, dimensional stability and moderate solidification temperatures, which provide significant energy and cost savings.
Zinc die cast parts are characterized by high performance, low production price, recyclability (providing they are not nickel and chromium plated) and non-toxicity. This material is also highly regarded in the automotive industry.
Despite this, some automotive manufacturers have started to pay increasing attention to polymers as an alternative to metals. However, zinc components remain irreplaceable in many applications, as reinforced polymers yet cannot match zinc cast alloys in strength and durability.
Polymers are very attractive because the injection molding process used to form parts is cost-effective. Nonetheless, some of the physical properties of zinc, such as its high heat conductivity, make zinc indispensable and irreplaceable by polymers in fields where safety is an issue of utmost importance, such as car door handles.
The main disadvantage of zinc application remains its susceptibility to corrosion in acidic, strong alkaline environments and in industrial areas. The corrosion products appear as white and bulky deposits on the surface. For this reason, the surface treatment of zinc components is a common practice. Among the various finishes, electroplated coatings play an important role.
Zinc die casting is well-known for its high-quality finishing characteristics, but unpredictable variations in the surface composition caused by alloying agents and morphological modification formed by the die casting process itself, may seriously affect the quality of the plating and consequently the performance of the surface treatment.
The impact of the material composition
Unalloyed zinc is a brittle and low-strength material, and therefore additional elements are required to obtain the desired characteristics. Among all types of zinc die castings, Zamak alloys, consisting of pure zinc alloyed with small amounts of aluminum, magnesium and copper, are often the preferred choice with their exceptional castability. Table 1 presents the specifications for the most popular zinc die cast alloys, according to ASTM B86.2
Table 1 - Overview of Zamak zinc die cast alloys. The composition and chemical quality of zinc alloys are controlled by international specifications, since mechanical properties can be affected by the presence of impurities.
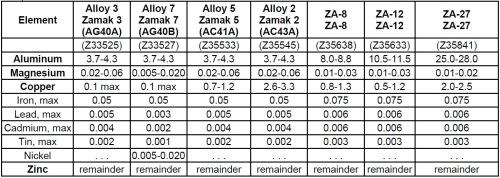
In the current research, samples of Zamak 5 were chosen as representative of this class of alloys. The addition of aluminum improves the fluidity of these alloys, and small amounts of copper increase the tensile strength and hardness,3 while magnesium confers higher resistance to intergranular corrosion that may occur in the presence of impurities.4 All of these agents are essential for achieving a high-quality die casting alloy, but they may also cause some negative side effects. For example, the presence of copper inclusions at the surface can cause galvanic coupling with zinc and aluminum, and since these two are characterized by a more negative potential than copper, their corrosion could then easily be promoted.
This phenomenon can be demonstrated by immersing the surface of the zinc die cast sample in a 0.5M sodium chloride solution and comparing the surface appearance before and after the exposure. The scanning electron microscopy (SEM) images in Fig. 1 show the initial intact surface of the sample (Fig. 1a), visible corrosion after 30 minutes (Fig. 1b) and clearly visible corrosion damage after one hour of exposure to the sodium chloride solution (Fig. 1c).
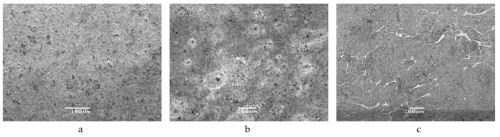
Figure 1 - SEM images of a zinc die cast sample surface (a) before and after immersion in a 0.5M sodium chloride solution for (b) 30 and (c) 60 minutes.
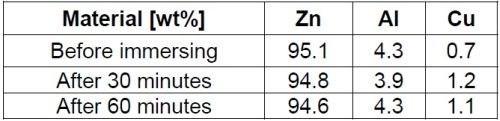
Energy dispersive x-ray spectroscopy (EDS) performed on the samples surface showed a decrease in the amount of zinc and aluminum and a concurrent increase in copper (Table 2). This was an example of selective corrosion, where zinc and aluminum were anodically dissolved, while the more noble copper inclusion remained at the surface as sites for the cathodic process of oxygen reduction.
Table 2 - EDS analysis results for a zinc die cast sample surface before and after immersion in 0.5M sodium chloride solution for 30 and 60 minutes.
From the results of this accelerated corrosion test, it is evident that non-plated zinc die cast parts must be stored in a humidity-free environment or covered with special protective additives in order to avoid mild corrosion of the part surface, which subsequently can have negative effects on the quality of the plating.5
A look at the die casting process from the plater’s point of view
Metal forming starts with the injection of the molten material into the mold cavity, which is essentially made of two steel dies. After the solidification time, the die opens and the casting is ejected. Due to the relatively low solidification range of the zinc alloys (379 - 390ºC), the zinc die casting process cycle is the fastest of virtually all other metal casting processes.
Solidification starts at the walls of the die and subsequently moves towards the bulk of the component. Consequently, the cooling speed differs; it occurs more rapidly closer to the die wall and slower in the inner volume, where cooling takes a longer time because of limited heat diffusion. This results in a variant structure within the component: a fine-grained skin, followed by a columnar zone with pronounced dendrites and an equiaxed zone in the bulk. According to the zinc-aluminum binary phase diagram (Fig. 2), the alloy solidifies in two different phases: the first is enriched with zinc and the second is richer in aluminum. inspection of the die cast samples cross-section (Fig. 3), shows the dendritic phase to be surrounded by the aluminum-rich phase. This observation is also confirmed by EDS analysis.
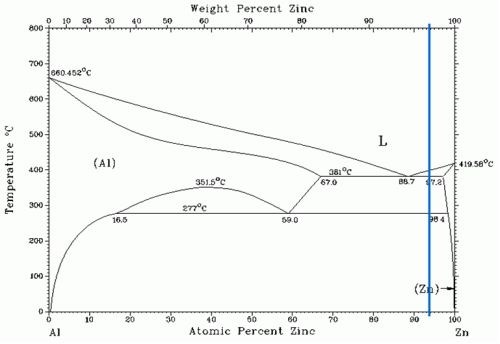
Figure 2 - Zinc-aluminum binary phase diagram. The blue line shows the zinc–aluminum ratio in Zamak 5 alloy.6
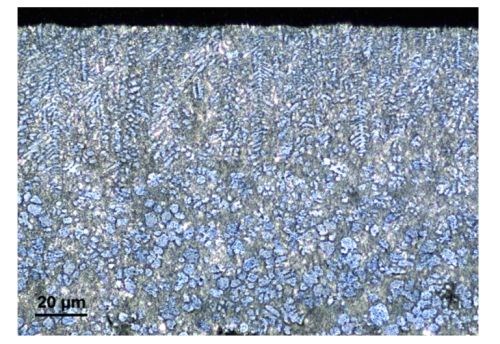
Figure 3 - Light optical microscope image of a cross-section of the die cast sample. Light blue zinc-rich zones are surrounded by an aluminum-rich gray matrix.
As mentioned earlier, this rapid solidification forms a surface layer characterized by a fine-grained structure and is completely free of porosity. This dense layer is relatively familiar to die casters and known as “die cast skin.” Usually the thickness of such a skin is between 50 and 300 μm.7,8
Zinc and aluminum are well-known chemically-active elements, especially in the molten state. These metals can react with moisture and other substances present in the mold, leading to gas formation, as demonstrated by the following reactions:
Zn + H2O = ZnO + H2 (1)
Zn + CO2 = ZnO + CO (2)
2Al + 6H2O = 2Al(OH)3 + 3H2 (3)
2Al + 3H2O = Al2O3 + 3H2 (4)
Hydrogen as a reaction product can be easily dissolved in the molten metal. Subsequently, when the melt starts to solidify, molecular hydrogen together with some trapped air forms bubbles, which remains in the bulk since the outer solid skin is already formed, resulting in an inner porosity. Therefore, die cast components are expected to show porosity in the bulk 50 to 300 μm from the surface, as can be observed in Fig. 4.
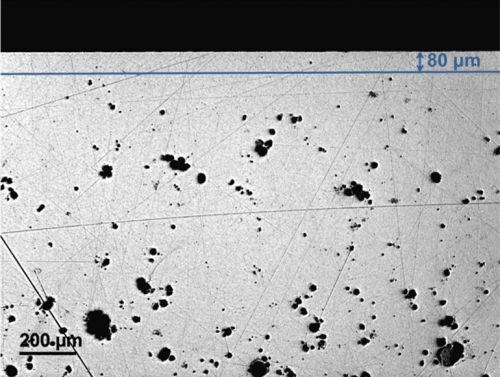
Figure 4 - Light optical microscope image of the die cast sample cross-section. The difference between the skin layer within the first 80 µm and the inner porous bulk is clearly visible.
Since mold cavities and dies are made of steel, the release and the following incorporation of impurities in the melt can seriously affect the quality of the die casted components. For this reason, barrier agents, called release agents, are applied on the die surface to prevent direct contact between zinc and steel. There are several types of die casting release agents: solvent- and water-based, carrier-free, sacrificial, semi-permanent and internal mold release agents. The most common used for zinc die casting are the first two categories. In these solvent- and water-based agents, carrier and organic substances are often present in the form of an emulsion, which, once applied, allows the carrier to evaporate and retain the organic substances on the surface. In contact with the metal melt, the die surface heats up and the organic substances are thermochemically decomposed. This phenomenon is known as cracking, where the organic substances undergo such reaction, if taking octadecane as an example:
C18H38= 18C + 19H2 (5)
This reaction shows the formation of carbon and molecular hydrogen: carbon continues to effectively act as a barrier for the melt, while hydrogen further contributes to the internal porosity.
In addition, in the case of excessive amounts of the release agent, measurable porosity can be present on the surface.9 Moreover, the formation of other substances could contaminate components and cause failures in subsequent surface treatment, if not removed by the post-molding processing. For instance, silicon contained in some of the release agents and thus incorporated onto the surface, can produce severe adhesion problems in subsequent processes such as plating, painting and gluing. Carbon inclusions present on the surface can act as inert sites for the cathodic oxygen reduction, as carbon has more a noble electrode potential than zinc, thereby increasing the probability of surface damage through selective corrosion. Die lubricant agents are not the only source of unexpected elements which can contaminate the surface of a zinc die cast component. Indeed, EDS analysis of the surface of an untreated zinc die cast surface shows an increased amount of aluminum and magnesium (Table 3), higher than the expected alloy composition (Zamak 5 alloy, Table 1).
Table 3 - EDS analysis results for the surface of an untreated zinc die cast sample.

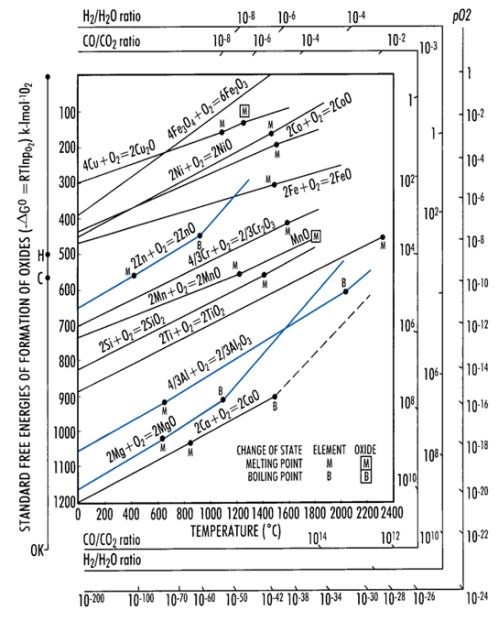
Figure 5 - Ellingham diagram for several metals giving the free energy of formation of metal oxides.10
Increased amounts of aluminum can be explained by the fact that aluminum has a high affinity to oxygen. As illustrated by the Ellingham diagram (Fig. 5), aluminum oxide formation is characterized by a negative value of Gibbs free energy, which validates the easy feasibility of the reaction. In addition, it demonstrates the high stability of the formed oxide. Hence aluminum oxide is immediately formed on the surface of the casting in accordance with the reaction:
4Al + 3O2 = 2Al2O3 (6)
Magnesium is also inclined to form oxides, but since its concentration is relatively low it does not produce such a critical outcome as compared to aluminum.
In the end, the desired components demonstrate somewhat unexpected results: an inner bulk material which hides considerable porosity, shielded by a thin and dense surface layer enriched with aluminum oxide and other elements often harmful to adequate quality in the plating process.
Required post-treatment operations
The post-treatment of die cast parts normally starts immediately after production in the casting house. It consists of cutting off runners and other excess material, a tooling process for shaping the components into the final form and grinding in order to remove all flashes and burrs from all the previous operations.
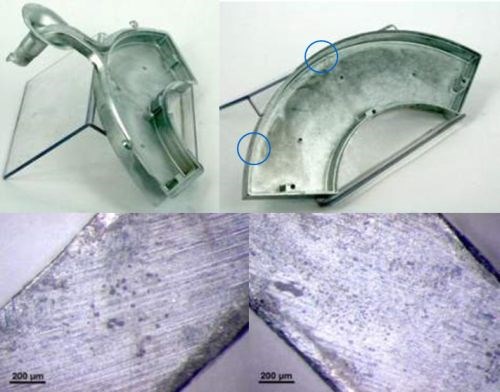
Figure 6 - Photos illustrating the pores revealed after machining operations.
Once the gating system is removed, the internal porosity on the cut-off edge is often revealed, as it is shown in Fig. 6. These emerged pores are areas with a high susceptibility for failures. Indeed, if these zones are not properly sealed before the plating process, the electrolyte can be retained by capillary forces which trap it under the coating. Since plating baths in most cases are aggressive media, a corrosion process can easily start and lead to blistering and coating delamination. Depending on the case and the duration of the process, these defects can be immediately noticeable after final inspection, or may appear after some time. Therefore, an accurate die design in which the runner gate is placed far enough away from the most decoratively valuable areas of the component is crucial.
The grinding process can be meant as a controlled form of corrosive wear: components can be tumbled in the presence of water, surface grinding compounds and abrasive chips. Chips and casted items are set in relative motion inside the machines working vessel, as presented in Figs. 7 and 8.
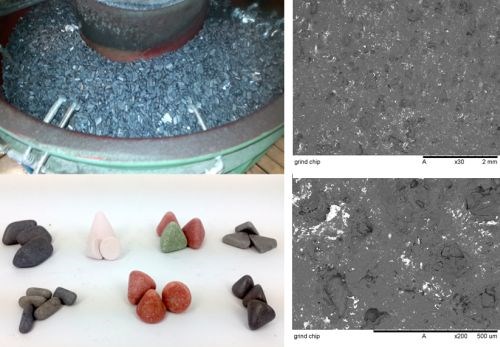
Figure 7 - Cast items and abrasive chips in the working vessel and different types of abrasive chips (left) and SEM images of the surface of grinding chips (right).

Figure 8 - Bowl vibrator for slide grinding at the die casting production site.
Currently, mostly ceramic or plastic bonded chips are used as abrasive chips. They are available in various sizes and shapes and vary in terms of specific weight, hardness and structural characteristics which confer them with different qualities. Depending on their physical properties, chips can be utilized for grinding, polishing or smoothing.
Due to these surface finishing processes most of the above-mentioned surface defects are removed together with a limited number of microns from the material. This truly is a tribo-corrosion process, which results in a damaged surface that can be seen from SEM images in Fig. 9. In order to avoid this phenomenon, die cast producers often add zinc corrosion inhibitors into the grinding solutions.
Comparing the results of the surfaces before and after grinding, the EDS analysis of the ground surface (Table 4) indicates a noticeable decrease in the carbon content. There is also a slight decrease in the oxygen content. We can assume that the grinding has mechanically removed the residues of the lubricating agents and the products of their thermal decomposition from the surface. Copper and carbon act as cathodic sites when the surface is wet during pretreatment steps, thus introducing selective corrosion, as previously mentioned above.
Table 4 - EDS analysis results for zinc die cast sample surface before and after grinding.


Figure 9 - Macro photos and SEM images of a zinc die cast sample surface before (left) and after (right) grinding.
Consequently, the grinding step is, without any doubt, an essential die casting finishing process and a fundamental pretreatment for plating.
It is worth mentioning that grinding and all of the mechanical treatments of zinc die cast components must be conducted with particular attention to the die cast skin, since this is the surface that provides the best results in further treatments, but only if it is kept intact.
Conclusions
The analysis of the impact of the material composition has revealed the necessity of storing non-treated zinc die cast parts under a controlled atmosphere in the absence of humidity, or better, to cover them with special protective greases in order to avoid mild corrosion of the surface. In this manner, the quality of the subsequent plating process is maintained within the product specifications.
A recommended practice is to plate the zinc parts immediately after the pretreatment since wet conditions accelerate the corrosion process on the surface and hence affect the surface quality.
Detailed consideration of the die casting process has demonstrated that reality always differs from expectations and very often the surface quality of components fails to live up to the requirements for plating. The presence of inner porosity in the bulk material, aluminum oxide on the outer layer and harmful impurities must not be ignored. The grinding process constitutes a valid pretreatment for plating, but as with all mechanical treatments, it must be held conscientiously in order to maintain the precious die cast skin, essential for the best results in plating and other subsequent treatments.
References
1. K. Miyoshi, "Solid Lubricants and Coatings for Extreme Environments: State-of-the-Art Survey," National Aeronautics and Space Administration, Springfield, 2007.
2. ASTM B86-13, Standard Specification for Zinc and Zinc-Aluminum (ZA) Alloy Foundry and Die Castings, ASTM International, West Conshohocken, PA, 2013; www.astm.org.
3. T. Savaşkan and M.Ş. Turhal, "Relationships between Cooling Rate, Copper Content and Mechanical Properties of Monotectoid-Based Zn-Al-Cu Alloys," Materials Characterization, 51 (4), 259-270 (2003).
4. K.A. Esakul, "Intergranular Corrosion Failure in Zn-Al Alloy Solenoid Valve Seats," Handbook of Case Histories in Failure Analysis, ASM International, Materials Park, OH, Vol. 1, 1992.
5. Z. Panossian and J.V. Ferrari, "Case Studies of Blistering Problems in Zinc-Plated Steel and Zinc-Plated Die-Cast Zinc Alloy Parts," Plating & Surface Finishing, 91 (9), 48-52 (2004).
6. Landolt-Börnstein Database, Binary Systems.
7. M. Gelfi, et al., "Microstructural and Mechanical Properties of Zinc Die Casting Alloys," Advanced Engineering Materials, 6 (10), 818-822 (2004).
8. W.T. Andresen, Die Casting Engineering: A Hydraulic, Thermal and Mechanical Process, Marcel Dekker, New York, 2005.
9. J. Titley, "The Role of Alloys and Melting Processes in the Cause and Elimination of Zinc Die Casting Defects," Global Casting Magazine, (2013).
10. L.S. Aubrey, "Method for Filtering Molten Aluminum and Molten Aluminum Alloys," U.S. Patent 8,486,176 (July 16, 2013).
About the authors

Valeriia Reveko is an R&D engineer in Collini GmbH, Austria, currently making an industrial Ph.D. project in materials science and surface technology at the Technical University of Denmark (DTU) under the supervision of Professor Per Møller. Valeriia holds an M.Sc. in technical electrochemistry and in her current research is developing protective-decorative nickel-like coatings for indoor use that are meant to limit human exposure to nickel.

Professor Per Møller has a Ph.D. in surface technology from the Technical University of Denmark (DTU), in Lyngen, Denmark. During the past 30 years, he has been engaged in contract research with industry covering nearly all aspects from micro-plating under cleanroom conditions to design and implementation of industrial-scale electroplating lines. He is author or co-author of more than 135 scientific papers and holds more than 25 patents in the field of surface technology and electrochemistry. Currently, he is Professor in corrosion and surface technology at the MEK-DTU Section for Materials and Surface Engineering. He is the 2017 recipient of the NASF William Blum Scientific Achievement Award.
*Corresponding authors:
|
Valeriia Reveko Collini GmbH Schweizer Str. 59 6845 Hohenems, Austria E-mail: vreveko@collini.eu |
Dr. Per Møller Technical University of Denmark Produktionstorvet, Building 425, 2800 Kgs. Lyngby, Denmark E-mail: pm@epc-info.dk |
Related Content
Advantages to Pumped Eductor Agitation
Not all agitation methods are created equally. Pumped agitation with eductor nozzles can improve process tanks and quickly show a reduction in operating costs while keeping staff safe, following environmental legislation and preventing pollution.
Read MoreTrivalent Chrome Overview
As the finishing industry begins to move away from the use of hexavalent chromium to trivalent chromium, what factors should finishers consider as they make new investments? Mark Schario, chief technology officer for Columbia Chemical offers a helpful overview of this complicated topic.
Read MoreAn Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreTroubleshooting Alkaline Zinc
One of the most common problems that can arise when plating with alkaline zinc is an imbalance of brightener in the solution. In this helpful Ask the Expert article, Chad Murphy of Columbia Chemical discusses how different zinc metal concentrations and brightener concentrations can impact efficiency.
Read MoreRead Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More





















