The Importance of Particle Size in Liquid Coatings
Coating problems and solutions associated with particle size reduction...
The paint industry has changed fromart to science in the last one hundred years. Today, paint technology uses the sciences of chemistry, physics and engineering. New technologies provide new materials to work with, new production equipment, processes and substrates. Unfortunately, growth always brings with it a certain number of problems.
Today's paint systems are often so complex and our technology so advanced that it is possible to overlook the exact nature of a problem. It is often assumed that the cause of a problem is chemical, when actually it may be physical.
Poor color strength or hue, as well as other properties such as opacity, transparency or gloss are often blamed on off-spec pigment. Many problems are not due to chemical reactions or poor raw material quality, but rather are due to particle size distribution.
The following properties are dependent on particle size:
- Flocculation
- Hue/Tint Strength
- Hiding/Transparency
- Gloss/Flatting and Film Appearance
- Viscosity
- Stability
- Weather Resistance
The degree to which these properties can be optimized is related to the dispersion process used, the energy expanded on the solid particles and the length of time the pigment particles are subject to the dispersion energy.
Flocculation, tint strength, hue and transparency. Paint chemists describe a variety of color-related problems caused by numerous mechanisms such as flocculation. The most common example is a coating pigmented with titanium dioxide and a colored pigment, especially an organic, will develop more color when the applied wet film is subjected to additional energy.
Normally, this type of effect is experienced when a white base is tinted with a dispersion of the organic or colored pigment. If the resulting mixture exhibits a color change due to additional shear, the usual conclusion is that the organic color pigment dispersion had already been flocculated or that it flocculated in the white tint base due to chemical inadequacies with respect to either the stability of the organic pigment dispersion or to incompatibility between the pigment dispersion and the white tint base. In reality, the rubbing often occurs because the organic pigment particles were not sufficiently deflocculated when originally dispersed. Organic pigments, as received, are in a flocculated state. High-speed mixing equipment does not have sufficient energy to break up even loosely packed organic pigment agglomerates. Therefore, if we add dry, colored, organic pigment to a white tinted base, the resulting finish will not only be grainy, but rubbing will indicate a tremendous difference in color development. The rubbing test, although crude, will subject the coating to a considerable amount of energy, even higher than what can be achieved with a high-sheer dispersion.
This is an extremely crude example, and we are all aware that these organic pigments must be predispersed in order to obtain desirable results. However, many of our problems are related to the fact that we qualify a pigment dispersion by relating it to a given Hegman grind reading.
The Hegman fineness of grind gage, although useful, is often misleading. The Hegman grind gage only indicates the size of the largest particles in a dispersion. It indicates nothing about particle size distribution. The state of deflocculation of the pigment is related to the particle size; and to obtain optimum results of no rubbing or deflocculation, the pigment must be dispersed as close to its ultimate particle size as possible.
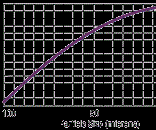
Tests made on Quinacridone Violet have shown that as the particle size is reduced, the amount of rubbing observed when tinting a white tint base diminishes, as indicated in Figure 1 (right). In Fig. 1, the degree of rubbing is expressed in a range of one to 10; 10 being severe and one showing no rubbing.
Tint strength. To further illustrate the effect of particle size reduction on Quinacridone Violet, dispersion was made to Hegman fineness of 7 NS. This is the point at which most paint chemists and production personnel would stop if they were going to tint an enamel having a Hegman grind of 7 NS; and also to a 7 3/4 NS, or almost off the gage. A white enamel was then tinted with these two dispersions, and comparisons were made of rubbing, tint strength and hue. The resulting comparison showed that the 7 NS dispersion rubbed up considerably, and was also 15% weaker in tint strength.
Transparency and hue. When these same two dispersions are compared in transparent films, large differences in transparency as well as hue are observed. These differences are extremely important when making metallic coatings. When these two dispersions were used to make a metallic lacquer finish, the results were dramatic. The paint produced with the 7NS dispersion showed poor metallic flip-flop, a dull, bluish washed-out film and lower gloss than the paint made with the 7 3/4 dispersion.
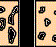
Photomicrographs of the transparent films show the distinct differences in particle size distribution. In addition, actual particle size distribution using a Coulter counter shows that the average particle size of the 7 NS dispersion was six microns, while the 7 3/4 sample was 0.5 microns.
The fact that most organic pigments undergo these significant changes in hue, tint strength and transparency, is not a new concept. Many coating manufacturers sacrifice the extra strength to avoid running longer grinding cycles or purchasing better equipment, not realizing that giving up 10% strength can be costly. Also, the ultimate quality of the finished product is sacrificed. Rubbing, transparency, clarity and brightness in metallics, as well as other properties are not achieved unless the pigment particle is reduced close to its ultimate size.
Hiding. Just as transparency is dependent on particle size reduction, hiding power is dependent on particle size. However, in the case of hiding it is necessary to control particle size within a given range.
Titanium dioxide is specifically processed to a particle size of 0.20 to 0.35 micron, or approximately equal to one-half the wavelength of light. By dispersing this pigment to its optimum size, ultimate hiding is obtained. There are other inorganic pigments that are by design opaque. Usually, the finer the particle size, the more opacity possible.
Inorganic pigment manufacturers have improved the dispersiblity of synthetic oxide pigments to the point that they are now promoting many of the pigments as stir-in or easy dispersing, meaning that a Hegman 6 NS or better can be achieved with high-speed dispersing equipment. While this is certainly true, hiding power is often sacrificed.
Typically, these pigments are red and yellow oxide. To illustrate the effects of particle size on hiding, several of the oxide pigments were dispersed using both high-speed dispersion to a 6+ NS Hegman (25 microns) and small media milling to a 7 1/2 NS Hegman (six microns or less). In most cases, a gain in hiding power was noted as the particle size was decreased to the approximate average size of the synthetic pigment crystal. When the results were compared, it was discovered that to reach equivalent hiding power, up to 15% more pigment was needed with the dispersion ground to only 6+NS.
When yellow oxide was dispersed further, a notable color shift took place, resulting in a dirtier color and a loss in hiding power. The severe discoloration indicates breaking or destruction of the original pigment particles.
When the synthetic iron oxide pigment is dispersed to a 6 1/2 NS Hegman (20 microns), the intended dark-red oxide or maroon-like shade is obtained. However, on further dispersing these grades, not only is there a definite improvement in hiding power, but there is a shift in both mass tone and tint to a medium-red iron oxide shade. The dramatic hue shift is due to the destruction of individual pigment crystals, except that in this case the pigment particles have been reduced to the size of the next finer stage of red iron oxide production.
While there are other parameters that contribute to hiding power, controlling the particle size will not only yield optimum hiding power, but also diminish costly reprocessing due to undesirable color drifts.
Flatting. In trade sales, as well as many industrial coatings, a variety of additives are used to control gloss. These materials include silica in clear coatings or varnishes and other extender pigments such as clays, talcs and carbonates in pigmented systems. While it is common knowledge that the solid particles or pigments in a given coating must be smaller in diameter than the dried film thickness of the intended coating, many people fail to realize that when using silica to lower gloss, precise particle size control is important.
How often has it been reported that the gloss of a batch of satin varnish was 10 units of gloss too high; and, after adding silica to correct it, it was 15 units of gloss too high?
| TABLE I - Glass rate and Particle Size | |
| Silica Particle Size in microns | Gloss of Varnish |
| 35 | 20 |
| 25 | 30 |
| 16 | 42 |
| 6 | 60 |
The reason is that while grinding in the silica, the silica already in the system was overground, resulting in a loss of flatting efficiency. Table I shows the gloss of several varnishes of exactly equal composition, except that the silica-flatting additive was dispersed to a different particle size.
Flatting, transparency and gloss all depend on scattering the reflected light. Once the flatting additive has been reduced beyond a given particle size, the film surface becomes more uniform. This allows the angle of incidence of more of the reflected light to be equal to the angle of reflection; therefore, gloss goes up.
Gloss. Since reducing particle size will reduce flatting efficiency, it is only natural to assume that it will benefit gloss. The particle size of the pigments in a coating will have an effect on film smoothness and cause scattering of light (Fig. 3).
Earlier, two dispersions of Quin-acridone Violet dispersed to different particle size ranges were shown to produce differences in color brightness. In addition, the one with the larger particle size violet pigment resulted in about 10 gloss units lower. When trying to produce jet black masstone glossy paints, the blackness of the resulting paint depends on achieving ultimate particle size distribution, especially since the blackest of the carbon blacks have perhaps the smallest particles of all pigments (about 0.07 micron).
Film Appearance. Finer particle size will yield better quality transparency and gloss due in part to film smoothness. In many coatings, especially industrial coatings, a variety of waxes, polyethylenes and other special hard polymers are used to impart certain properties to the cured film. These properties include lower coefficient of friction, improved mar and scuff resistance and reduced metal marking. This is accomplished without adversely affecting other properties such as recoatability, adhesion, gloss or cratering. Since these materials function by coming to the surface of the cured film, much of their beneficial and adverse effects are dependent on particle size.
Since the action of these particles is at the surface of the film, it is obvious that the larger the particle, the greater the effect on film uniformity. This is especially true in baked coatings where these particles become liquid during the baking cycle. In their molten state, they are usually not compatible with the coating's resin and/or vehicle system. Therefore, film smoothness is influenced by differences in the surface tension of the two liquids.
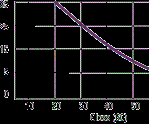
Figure 3 (at right) shows what happens in a coating containing large polyethylene particles in the various stages of film formation. After application and prior to baking, the solid polyethylene particles near the surface create a slight disruption of the film smoothness and cause the paint film wall above it to thin by drainage.
During baking, the polyethylene melts and becomes a liquid. Due to differences in surface tension, the polyethylene will cause the coating liquid to push away from the polyethylene droplet, forming a crater. Upon drying and cooling, the polyethylene droplet reverts back to a solid and, while undergoing some shrinkage, remains as a ball.
Naturally, the larger the particle, the larger the crater. This affects film integrity, transparency and gloss in clear coatings, as well as reflected image in pigmented coatings. Normally, this can be controlled by reducing the particle size, which not only reduces film imperfections, but also increases the activity of the additive used.
Rheology and stability. Paint production usually uses predispersed pigment concentrates, whether produced in house or purchased from an outside vendor. Because these concentrates are used in a variety of chemically different coatings, broad compatibility is naturally desirable.
To achieve this effect, the manufacturer of these concentrates tries to keep the formulation as simple as possible in order to prevent adverse effects due to the use of unnecessary additives. In addition, these color concentrates are often maintained in stock for a long time and, therefore, must show good storage stability.
Many paint chemists believe additives should sometimes be used just in case. An example is with rheology modifiers or thickening and anti-settling agents. Often this is done without a clear understanding of whether the additive is really necessary; other ways to achieve the required results; or what will be the adverse effects on aging.
With predispersed color concentrates there are two types of stability required: 1) Resistance to settling on aging; and 2) Resistance to flocculation on aging.
Before introducing additives that could adversely influence properties such as water resistance, broad-range compatibility, flocculation, flow and leveling, the first parameter that should be evaluated is the dispersion stability as a function of particle size.
The stability of pigment dispersion is improved through particle size reduction in three ways:
- Rheology and/or viscosity. Reducing pigment particle size increases pigment surface area, which usually results in increased viscosity. Also, many organic and even inorganic pigment systems develop a thixotropic state. Higher viscosity or induced thixotrophy prevents pigment mobility, preventing both settling and reflocculation.
- Prevention of flocculation. In floc-culation, pigment particles tend to reagglomerate, resulting in a loss of color intensity and poor color uniformity. Reducing the particle size will prevent reagglomeration. Reducing the particle size increases the viscosity of the system; it further reduces the possibility of migration.
- Settling. One of the main stability concerns of most coating manufacturers is settling. Differences in particle size can show widely varied results in settling of a spherical object in a liquid medium, and apply it to the rate or settling of a pigment particle in a dispersion or coating system. We can see the value gained in stability due to simple particle size reduction.
Chalking, lightfastness and weather resistance. In addition to package stability, the stability of the cured film, or the performance of the coating after it has been applied and cured, can be affected by pigment particle size.
Opaque coatings require a variety of pigments that, depending on their selection, can affect coating properties. In many cases, the selection of the pigment is based on aesthetic or economic values, and little thought is given to its actual properties in relation to the required coating properties or the effects of particle size on those properties.
With systems of borderline properties, chalking, lightfastness and gloss retention can be adversely affected by particle size reduction. Simple experiments conducted with a general-purpose grade of titanium dioxide in combination with both synthetic red iron oxide and yellow iron oxide pigments dispersed to different particle size ranges, resulted in considerable difference in water resistance, gloss retention and chalking. The lightfastness of some borderline pigments is also equally affected by differences in particle size distribution.
To show some of these effects, enamels based on exactly the same vehicle type and pigment ratios were prepared and dispersed into differing particle size ranges. In one case, the pigment consisted of a 2:1 ratio of titanium dioxide and yellow iron oxide; and in the other case a 2:1 ratio of titanium dioxide and red iron oxide.
Paint was prepared in both colors at an average particle size range of 25 microns and six microns. The finer particle size, as expected, produced a higher gloss in the unexposed panels. Five hundred hours of QUV exposure resulted in the apparent gloss and color changes as shown in Table II
| TABLE II - QUV Exposure Results | ||||
| Yellow Oxide/TiO2 | Red Oxide/TiO2 | |||
| 25µ | 6µ | 25µ | 6µ | |
| Gloss (Unexposed) | 91 | 94 | 92 | 94 |
| Gloss (Exposed) | 70 | 52 | 72 | 53 |
| Loss of Gloss on Exposure | 21 | 42 | 20 | 41 |
| % Strength Lost on Exposure | 3.5 | 7.0 | 4.0 | 7.0 |

|
1.43 | 1.85 | 1.05 | 1.45 |
Reduction in particle size causes an increase in pigment surface area. Therefore, the coating with pigment reduced to six microns will have more pigment exposed on the surface, with less vehicle to protect against deterioration. Since the titanium dioxide chalks faster than the iron oxides, the result is an apparent loss of strength on aging. After washing the exposed area and comparing it to the unexposed area, the results showed that the two surfaces were of the same gloss and color, indicating the changes in the above numerical results were due strictly to chalking. These results were obtained by using pigment combinations that would normally be expected to chalk. Selecting pigments that would be more resistant to chalking or less sensitive to light would reduce the effects indicated. However, when relative water resistance of these same coatings was evaluated, the results were opposite.
The paints having the finer particle size distribution ranges showed considerably better water resistance. The reason can be explained by the fact that in these experiments, the pigment combination was purposely chosen from those pigments that are perhaps the most hydrophilic in nature. In Figure 4 (at right), which schematically represents the two coatings containing different particle sizes, we can see that the larger particle size would tend to provide a wick effect through which moisture could travel to the substrate. This results in blistering, softening and eventual destruction of the coating and corrosion of the metal substrate. Even though we have achieved technical excellence in polymer technology that provides us with coatings capable of beautifying, protecting, insulating and even reacting with a given substrate, many of the properties can be lost if we do not pay attention to the size of the solid particles within a coating film.
Adapted with permission from Paints and Varnishes, Milan, Italy.
GLOSSARY
Flatting: Undesirable loss of gloss during drying.
Flip-flop: Decorative effect with metallized finishes in which there is a difference in color depth and flash when viewed at different angles.
Hegman Fineness of Grind: Device that measures the fineness of dispersion of a pigment based.
Quinacridone violet: Versatile member of the quinacridone pigment family. Used as a toning pigment; to neutralize yellow tones in white; and used in blends to obtain bright reds. Highly transparent.
Rubbing: Procedure for testing the ease with which a lacquer or enamel can be rubbed to a smooth high-gloss finish.
Related Content
The Value of Robotic Paint Performance Testing
Considerations for implementing the use of automation for paint performance testing.
Read MoreProducts Finishing Reveals 2024 Qualifying Top Shops
PF reveals the qualifying shops in its annual Top Shops Benchmarking Survey — a program designed to offer shops insights into their overall performance in the industry.
Read MoreCoatings Plant Evolves with Market Trends
Expanding its focus from exclusively serving the RV industry, one of this company’s stand-alone coatings plant has successfully extended its services to additional markets.
Read MoreHenry Ford Is Still Right When It Comes to Color
Who would have imagined that more than 100 years after his famous statement about any color as long as it’s black would still have relevance of a sort?
Read MoreRead Next
Education Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More