Understanding and Managing White Spots on Anodized Aluminum
Having trouble with spotting defects when anodizing? Taj Patel of Techevon LLC offers a helpful overview of the various causes of white spots and potential solutions.
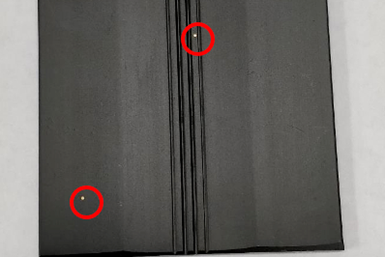
Photo Credit: All images courtesy of Techevon LLC
Q: What causes white spots when anodizing and how can I prevent them?
A. White spotting/staining is a common phenomenon, however, understanding the cause of these surface defects can be challenging. The primary source for this confusion is that white spotting is not caused by the same issue all the time and in many cases, it could be a combination of multiple factors that result in the surface defect. Furthermore, some defects are related to the actual anodic coating, whereas others could simply be on the surface of the coating. In the list below, we have summarized the key properties of the anodizing process that can lead to white spots or other surface defects:
- Alloy type
- Machining quality/part profile
- Pretreatment process
- Orientation/airflow on production line
- Anodizing conditions
- Coating passivation
- Rinse cycle
- Dye conditions
- Sealing process
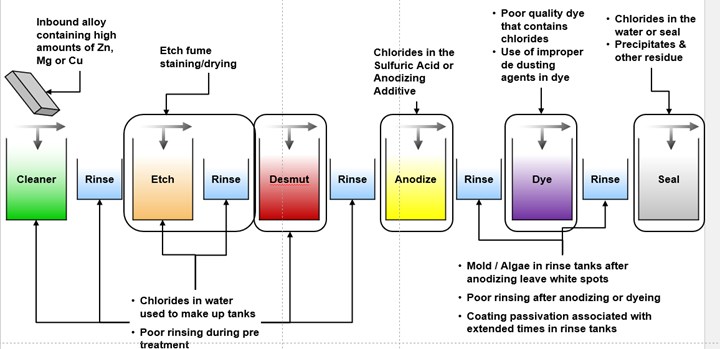
This graphic shows the multitude of different process tanks and areas that could result in surface defects that look like white spots.
Alloy Type
Sources of spotting/staining include the following:
- Certain alloy constituents are more susceptible to galvanic activity than others (2000, 7000)
- Alloys with high levels of Cu, Mg and Zn
- Rinse water corrosion (Chlorides and pH)
- Chlorides in dye tank
Solving the problem starts with understanding the alloy type. Potential solutions include managing rinsing (reducing immersion time, improving water quality), using sacrificial anodes (magnesium bars), chloride management (determining source and containing and reviewing tooling (Al vs Ti).
Machining Quality/Part Profile
Machining quality and part profile can also play a role in spotting defects. Sources of spotting/staining can include sharp edges on parts, contaminated cutting fluid and poor material handling procedures.
Potential solutions include:
- Checking parts for sharp edges
- Testing cutting fluids for algae/mold that can cause corrosion
- Using sacrificial anodes (magnesium)
- Chloride management — source and contaminant
- Review tooling (Al vs. Ti)
Pretreatment Process
Sources of spotting/staining pertaining to pretreatment include threaded blind holes (the smaller the hole the greater the problem) and poor rinsing, which contribute to the entrapment of process chemicals.
Potential solutions include eliminating pretreat (if possible), physically rinsing every hole on a load, and the use of special rinse aids in the tank.
Orientation and Production Line Airflow
When an anodizing load comes out of the tank at the same time as an etch load, caustic fumes can deposit on the anodized surface, particularly if air flows from the etch tank. Etch fume staining occurs when etch dries on the part prior to rinsing and usually results in spotting on the top of the load instead of the bottom.
Potential solutions include:
- Making sure the etch load and anodize load do not come out simultaneously
- Reversing airflow to go from anodization tank to caustic tank by using industrial fans
- Reducing time between etch and rinse or dwell time over etch tank
- Checking etch chemistry
Coating Passivation
Sources of spotting/staining:
- Extended immersion times in rinse water (greater than 10 minutes)
- Rinse water contamination
- Inefficient scheduling/planning
Potential solutions:
- Reduce immersion times in rinse tank
- Make sure the parts are rinsed well through higher frequency immersions for shorter durations
Rinse Cycle
Sources of spotting/staining:
- Insufficient rinsing after anodizing
- Poor rinse water quality
- Certain alloys are more susceptible to poor rinsing
Potential solutions:
- Improve rinse process using a spray rinse or higher frequency immersions
- Use nitric rinse if possible
Dye Conditions
Sources of spotting/staining:
- Use of improper dedusting agents in the dye
- Chlorides in dye
- Chlorides in water
- Algae contamination
Potential Solutions:
- Make sure to purchase dye with proper dedusting agents
- Use algaecides where possible
- Contact supplier to determine if chlorides are present in dye
- Adjust pH and temperature
Sealing Process
Sources of spotting/staining:
- Chloride contamination
- Precipitated salts
- Surfactant drying
- Algae residue
Potential solutions:
- Check water quality
- Review temperature of seal chemistry
- Check pH of seal
Surface Defect Sources
In many cases, surface defects may exist on aluminum which can become more visible after anodizing, resulting in “white spots.” Below is an overview of potential surface defects to be watchful for.
Galvanic Corrosion
Galvanic pitting occurs when dissimilar metals are electrically connected and the less noble of the metals corrodes. Particularly at sites where there are distinct breaks of the alloy constituents in the anodic coating. Galvanic activity can happen in several different process tanks and is random across a part which makes it difficult to identify. Several factors can cause galvanic activity:
- Alloy type (1100, 2000, 5083 and 7075).
- Sharp machined edges
- Chemistry contaminants
- Extended immersion times
- Titanium tooling
Atmospheric and Other Corrosion
Atmospheric or fallout corrosion can occur on incoming metal as a result of poor material handling. Fallout corrosion typically occurs in specific areas of the part (outside surface) instead of the entire part. There are several factors that contribute to fallout corrosion, including contaminated cutting fluid (i.e., algae), and moisture in part storage areas.
Water Contaminants
The two primary water contaminants to be aware of are chlorides and algae. Chloride levels can vary significantly depending on the source of water; therefore, using DI water for all tanks after the pretreatment will significantly reduce the likelihood of chlorides. Additionally, chemistry should be checked for chlorides.Contact your vendor to understand what chlorides if any could be in the following products:
- Anodizing additives
- Post-anodize rinse additives
- Dyes
- Seals
Algae is typically linked to dyes because many dyes are consumed by algae. Therefore, dye tanks should be regularly dosed with algaecides, particularly in the summer months. Furthermore, when storing dyes for extended periods of time, be sure to follow supplier guidelines on storage. In many cases, liquid dyes are more susceptible to algae contamination.
Caustic Fume Staining
Caustic fume staining occurs when parts are removed from the etch tank and are exposed to etch fumes for extended periods or when the etch dries on the part without immediate rinsing. There are several factors that can contribute to caustic fume staining/drying:
- Crane lift time
- Ventilation/airflow around etch tank
- Timing between etch and first rinse
- Etch chemistry
Bleed Out
Bleed out occurs when hot etch solutions cause threaded holes to expand and trap caustic, which carries over to till the next hot process tank, which is the dye tank. When the etch “bleeds out” it creates white stains that do not take color. There are several factors that can contribute to bleed out:
- Part orientation
- Rinse cycle and frequency
- Rinse aid chemistry
Precipitates/Solids Residue
Precipitates and other solids residue on a part are most often seen during the final stages of the anodizing process, such as dyeing or sealing. There are several factors that can cause precipitates or other residue:
- Nickel hydroxide precipitate from seal
- Surfactant drying from seal
- Algae residue from dye or rinse tanks prior to seal
Nickel hydroxide formation can be linked to a high pH (>6.0). When this happens, the nickel hydroxide precipitate forms and cannot go back into the solution.
For more information, including images of these defects, contact Tej Patel via email Tej.Patel@techevon.com.
About the Author

Tej Patel
Tej Patel is the director of Techevon LLC. Visit techevon.com. In addition, read Patel’s award-winning paper presented at the 2022 Aluminum Anodizers Conference:
short.pfonline.com/UVanodiz
Related Content
How to Maximize Nickel Plating Performance
The advantages of boric acid-free nickel plating include allowing manufacturers who utilize nickel plating to keep up the ever-changing regulatory policies and support sustainability efforts.
Read MoreSolvent Versus Aqueous: Busting the Myths
Is aqueous cleaning “greener” than solvent cleaning? Is solvent a more effective cleaner than aqueous? These and many other questions are answered here to debunk the misconceptions that many manufacturers have held onto for years.
Read MoreAnodizing for Bonding Applications in Aerospace
Anodizing for pre-prep bonding bridges the gap between metallic and composite worlds, as it provides a superior surface in many applications on aluminum components for bonding to these composites.
Read MoreTop Reasons to Switch to a Better Cleaning Fluid
Venesia Hurtubise from MicroCare says switching to the new modern cleaning fluids will have a positive impact on your cleaning process.
Read MoreRead Next
Techniques to Enhance UV Stability of Dye Anodized Coatings
The ultraviolet stability of anodic coatings is assessed based on lightfastness/colorfastness testing.
Read MoreFixing Corrosion Between Anodized Aluminum and Steel
Anne Deacon Juhl, Ph.D., with AluConsult, says Galvanic corrosion is due to an electrical contact with a more noble metal or a nonmetallic conductor in a conductive environment.
Read More



















