Understanding the Anodic Oxide Finish – The First Commercially Available Nanoscale Coating
Dr. Jude M. Runge is the 2020 recipient of the NASF William Blum Scientific Achievement Award. The COVID-19 crisis and the cancellation of SUR/FIN 2020 have affected the timing of the formal award and her delivery of the William Blum Lecture. To honor her properly now, it is timely to publish one of her earlier papers, showing the caliber of her work. Here, she notes that nanomaterials, nanotechnology and nanoprocessing are the marketing buzzwords for the 21st century, stressing that, after all, conventional anodizing processes yield a nanoscale finish. Armed with this knowledge, the ease, reliability and reproducibility of the anodic finish enable insights into new applications.
by
Dr. Jude M. Runge*
CompCote International, Inc.
Elmhurst, Illinois, U.S.A.
Technical Editor’s Note: This past summer, the COVID-19 crisis caused the NASF SUR/FIN Conference to be cancelled, something that only World War II had done, and just in 1945. Nonetheless, events failed to deter the annual selection of NASF Awards. In particular, the NASF was pleased to announce the 2020 recipient of the NASF William Blum Scientific Achievement Award - Dr. Jude M. Runge. Unfortunately, due to the timing, Dr. Runge will not deliver her William Blum lecture until SUR/FIN 2022. To honor her properly now, it is timely to publish one of her earlier papers, previously published as Journal of Applied Surface Finishing, 2 (1), 47-55 (2007), showing the caliber of her work in meriting the award. Here, she challenged us with the premise that the decades-old process of anodization had been giving us nanoscale coatings all this time. And indeed that has been the case, as she described the nature of the pores developed in the coating. This paradigm-shifting revelation challenges the imagination in what can no longer be considered a staid, old mature industry. Armed with this knowledge, the ease, reliability and reproducibility of the anodic finish enable insights into new applications. A printable version of this paper is available by clicking HERE.
ABSTRACT
Just go to Google™ – Nanomaterials, nanotechnology and nanoprocessing are the marketing buzzwords for the 21st century. Did you know that conventional anodizing processes yield a nanoscale finish? Armed with this knowledge, we can approach our old industry with new enthusiasm. The long standing successful track record for the ease, reliability and reproducibility of the anodic finish should enable insights into new applications. Comprehensive comparative studies of various anodic oxide microstructures and their corresponding engineering properties enabled new scientific insight for the mechanism of anodic oxide formation on aluminum and aluminum alloys. The results of these studies indicate the importance of understanding the anodizing process and the synergy between process, microstructure and engineering properties to bring innovation and improvement to our mature industry.
Keywords: anodization, nanomaterials, nanotechnology
Introduction
The history of anodizing, the electrochemical oxidation of aluminum, dates back to the beginning of the last century. In fact, Bengough and Stuart’s patent in 1923 is recognized as the first patent for protecting aluminum and its alloys from corrosion by means of an “anodic treatment.”1 In 1936, electrocoloring the anodic oxide finish was invented by Caboni.2 Continued research through the 1960s, with the development of the scanning electron microscope (SEM), led to a more detailed understanding of the anodic oxide structure as a hexagonal close-packed (HCP) structure described as consisting of a porous layer and a barrier layer.3,4 See Figs. 1 and 2.
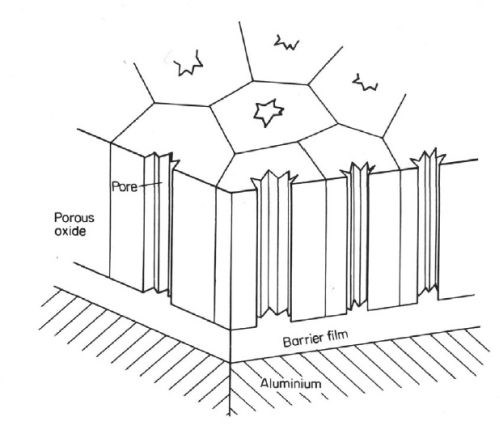
Figure 1 - Schematic of the anodic oxide layer published in Von Fraunhofer.3
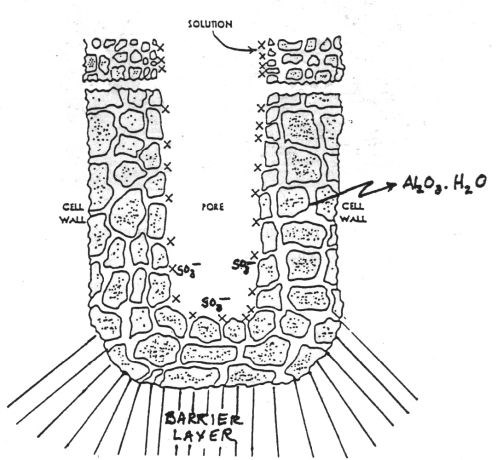
Figure 2 - Schematic of the anodic oxide presented in the Clariant Anodizing Class Notebook from 1991 shows the progress in the understanding of the structure.4
With the advent of the transmission electron microscope (TEM) in the 1970s and into the 1990s, an even deeper understanding of the porous aluminum structure as well as compositional and theoretical models of formation mechanisms was developed. All encompassed a structural concept of a barrier-type oxide in conjunction with a porous-type oxide. Review of these publications discloses the dimensions of the geometric features of the anodic oxide finish as nanoscale (10-9 m) and distinguishes between barrier type oxides and porous type oxides by process and electrolyte, yet persists in the theory that the porous finish develops from the formation of a barrier oxide on the entire substrate area, without regard to surface reconstruction, nucleation and growth of individual corrosion cells during oxidation.5 See Fig. 3.
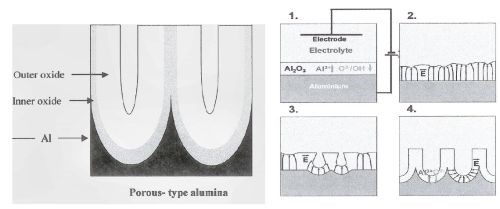
Figure 3 - A more current schematic of the anodic oxide presented by UMIST (Thompson and Wood). Pore formation schematic describes process as a function of both field enhanced and/or temperature-enhanced dissolution.5
Nevertheless, the long standing successful track record for the ease, reliability and reproducibility of the anodizing process has led to the use of the finish in many high-tech applications. Nanotubes and nanowires from cobalt, nickel and carbon fabricated from anodic aluminum oxide arrays have been researched and produced for the computer, energy and aerospace industries.6,7 Numerous other research groups, not mentioned specifically in this paper, are also using anodic aluminum oxide because of its self-assembling nanostructure.
Much of the research mentioned above has gone forward without realizing the existence of the commercial light metals industry. And, with the introduction of other nanoscale coatings to the market, e.g., sol-gel processes and other self-assembling monolayer processes, the image of anodizing as a mature, 85-year old process persists throughout industries that utilize aluminum components – anything but high-tech!
It is the responsibility of the metal finisher to recognize what has been produced through standard aluminum anodizing processes all along – the first nanoscale finish that yields consistent surface protection and durability for aluminum substrates. It is also critical to understand the hows and whys of the finishing process so the finish characteristics produced with standard parameters are understood yet can be changed to vary and improve them.
Through the course of our work beginning in the late 1990s and continuing through 2001 at the University of Illinois at Chicago, Saporito Finishing in Cicero, Illinois and Bodycote Surface Engineering in Kaufbeuren, Germany,8-10 and continuing work today at CompCote International, Inc., we have learned much about the anodic oxide finish as it applies to various anodization processes, parameters and electrolytes. As a result, we developed a new theory for porous oxide formation which challenges the convention that dissolution is the dominant mechanism for the porous oxide structure and presents solid corrosion theory as the source for the self-assembling nanoscale structure of the anodic finish.
Scientific characterization
Transmission electron microscopy (TEM)
Ultramicrotome sections of conventionally processed and unsealed Type II (commercial anodized) and Type III (hard anodized) aluminum alloy substrates were prepared parallel to the direction of finish growth. The sections were further prepared for TEM by way of precision ion processing (PIP). The finish sections were imaged and analyzed by way of TEM with electron energy loss spectroscopy (EELS).
Images of the Type II and Type III anodized finishes disclosed they were comprised of several close-packed unidirectional columns with closed, rounded bottoms. The number of columns, pore diameter and column wall thickness was consistent for each finish type but varied with process type. The Type II finish was comprised of many fine columns, each with a uniformly small pore diameter. The pore diameter measured approximately 5 to 10 nm and the column walls approximately 10 to 15 nm thick (approximately 25-nm pore center-to-pore center distances). See Fig. 4.
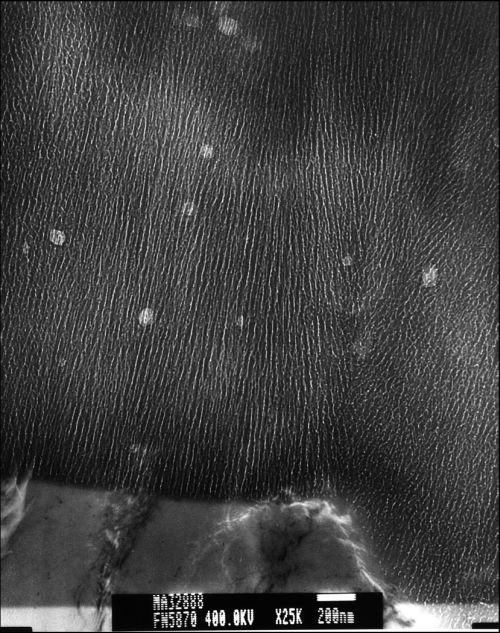
Figure 4 - Representative TEM micrograph of a Type II anodic oxide finish.
The Type III finish exhibited fewer columns with wider pore diameters and thicker column walls. The pore diameter measured approximately 30 nm and the column walls approximately 25 nm thick (approximately 80 nm pore-to-pore distances). A feature more easily discernible on the Type III finish was that of a distinct boundary line between each column. The boundary lines exhibited the same unidirectional character as the central pore and appeared to “knit” the individual columns together. These came to be called “knit lines” in our research and their location and appearance were important in developing the theory of film formation presented in this paper. See Fig. 5.
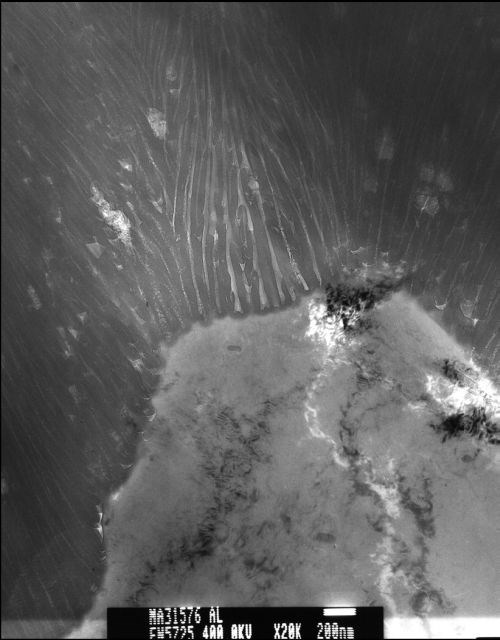
Figure 5 - Representative TEM micrograph of a Type III anodic oxide finish.
TEM also disclosed that while the anodized finish exhibited an ordered appearance, interfacial disorder was observed at the substrate in areas where the anodic oxide intersected grain boundaries, dislocations and inclusions. Disorder was identified as an apparent increase in column bases in the interfacial defect areas, increased outgassing (entrapped gas bubbles within the finish) and deviation from the perpendicular orientation of the finish to the substrate. Recovery of the finish growth was noted as additional growth fronts were observed below the disordered areas. See Fig. 6.

Figure 6 - Documentary TEM micrograph of a Type III anodized aluminum substrate. Note the presence of circular inclusions (iron-chromium) which were taken up into the Type III columnar structure during anodization. Atomic level defects such as grain boundaries and dislocations in the aluminum substrate also affect the finish structure at the finish-substrate interface (15,000X).
Chemical analysis via EELS and imaging by way of diffraction contrast showed conclusively that the finish is comprised primarily of amorphous, disordered, hydrated aluminum oxide, that is, aluminum hydroxide. No other metal oxides were identified within the finish that would indicate that alloy additions such as copper or silicon participate to become part of the anodic oxide finish. The anodic oxide finish, regardless of type, is amorphous and doesn’t exhibit diffraction contrast necessary to identify the oxide phase as corundum, α-alumina, Al2O3. Furthermore, related research has disclosed the only crystalline anodic finishes are those formed by way of spark anodization, where the crystalline phase of alumina formed was determined to be γ-alumina.11
High intensity infrared (FTIR) spectrographic analysis
TEM sections of a Type II unsealed anodic finish, of a Type II sample anodized utilizing an electroactive polymer additive to the electrolyte and a hot water-sealed Type III finish were analyzed by way of high intensity Fourier transform infrared spectrographic analysis at the Brookhaven National Laboratory in Brookhaven, NY, where the energy source for the instrument was hooked up to the synchrotron light source. The resolution of the instrument was 4 to 5 μm. Sections of each finish were analyzed using a 4 × 30-μm aperture. Data were collected from the aluminum substrate - anodic finish interface (bottom), the finish center and the top of the finish. The finishes measured 20 to 25 μm thick.
The trends in the infrared data collected from the bottom of the finish to the top indicate a shift in the amount of active hydroxide and sulfate groups. At the bottom of the finish, the Al-O feature at approximately 750 cm-1 dominates the spectrum, but shifts upward in the spectra at the center and the top as the sulfate peaks at 1000 to 1100 cm-1 become larger and more defined. With the development of sulfate absorption in the spectra, hydroxide absorption becomes pronounced. This makes sense as the surface of the anodic finish, regardless of type or formulation should exhibit more hydration at the interface where it contacts the environment.
The sample anodized with the electroactive polymer additive exhibited evidence of inclusion of the electroactive modifier with absorption in the higher IR. The spectral shifts toward the higher IR were noted on comparison to finish spectra obtained closest to the substrate, where the inorganic absorbances were most pronounced, to spectra obtained from the middle and finally the surface portions of the finish where absorption of both OH-1 and carbon-based inorganic salts were detected within the finish anodized with the electroactive polymer additive (composite finish). See Figs. 7 and 8.
A hot water-sealed Type III finish was processed within argon plasma. With increased exposure time within the plasma, a residue developed on the surface of the finish. Infrared analysis of the residue determined absorption characteristics for hydrated aluminum sulfate and/or aluminite:
Al2(SO4)•18H2O and/or Al2(SO4)(OH)4•7H2O.
The significance of these results are in the formation of a compositional as well as a concentration gradient across the anodic oxide finish from the substrate interface to the finish surface and imply that the same gradients exist from the knitline to the wall of the central pore. The results show that portions of the finish that remain in contact with the electrolyte, in addition to hydration, will also adsorb active counter-ion species from the electrolyte which further impact the function of the pore during finish formation and during subsequent processing.
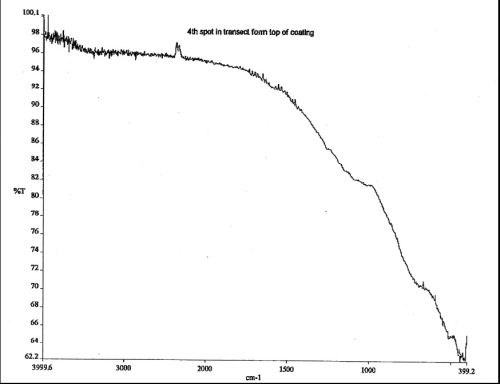
Figure 7 - IR spectrum of the composite finish adjacent to the aluminum substrate is identical to a conventionally anodized Type II film. The low end absorbance is typical for inorganic species.
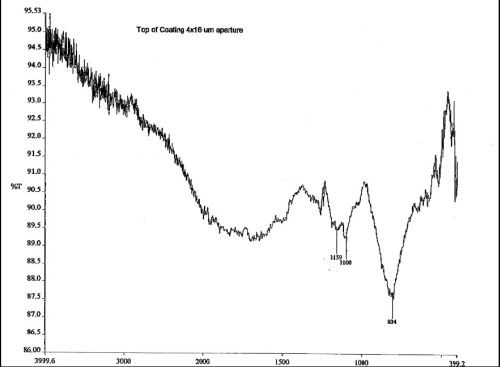
Figure 8 - IR spectrum of the composite finish exhibits a shift in the inorganic spectrum toward the more chemically reactive species of sulfate and hydroxide. Organic absorbances are also present in the higher IR indicative of inclusion of the electroactive polymer additive as well as more hydration.
X-ray photoelectron spectroscopy (XPS)
XPS studies were performed at the Technical University of Linköping in Linköping, Sweden. Samples were prepared following conventional anodizing process parameters (Type II). Additional samples were prepared that were anodized utilizing an electroactive polymer additive to the electrolyte processed at Type II parameters and anodized for an extended exposure time. The samples were anodized and mechanically removed to sealed containers to prevent surface contamination through handling or by ambient air. This insured that the analysis results would reflect the actual anodized surface composition from a depth of 0 to 30 Angstroms.
The Type II and sample anodized with the electroactive polymer additive for the 60-min exposure time exhibited the presence of sulfur as sulfate. Additional oxygen and aluminum was also detected. The high-level binding energy component for oxygen corresponded to H2O and OH-1, which supported the infrared data. The aluminum detected in the film surface of these samples was not metallic in nature. The low binding energy component was typical for disordered aluminum oxide. The sample anodized with the electroactive polymer additive did show chemical inclusion of the polymer. Saturated and π - conjugated carbons were noted with distinct linkages to the disordered (hydr)oxide structure as HO-C=O, O-C=O and C-OH.
XPS analysis of the extended exposure samples determined strikingly different surface constituents. More of the electroactive additive was detected in the surface of these samples. No sulfur in any form was detected. Evidence of metallic aluminum, aluminum as Al2O3 and copper as Cu2O was also identified. The results were important because they indicated these species were actually deposited from the electrolyte and not a function of the anodization of the substrate.
Atomic force microscopy (AFM)
Preparation and characterization of porous oxide anodized finishes on aluminum by Wang, et al. at the Argonne National Laboratory in Argonne, IL6 confirmed the porous oxide finishes were nanoscale. Although the purpose of the research performed was to utilize the finish as a template for nanoscale wires and tubes for electrical, magnetic and photonic applications, their work also documented clearly the nature of the porous oxide finish as a self-assembling array of individual columns.
Atomic force microscopy (AFM) techniques clearly document the anodic porous oxide finish as consisting of several columns per grain aluminum, self-assembled in an array not unlike a simple bubble array, hence the hexagonal arrangement. One of the samples, a coupon manufactured from 1100 aluminum alloy, was anodized at 0°C for 3 hr at 30 VDC in oxalic acid. The unit column per grain density of this sample, for an average substrate grain size of 2.5 μm, averaged 200 columns per grain. The pore diameter for this sample was 40 nm and the pore-to-pore central distances measured 100 nm.
In general, for all samples anodized, the column sizes and spacing varied, depending on the current density and electrolyte utilized. They ranged in size for pore-to-pore distance from 51 nm for lower current density finishes to 100 nm for higher current density finishes. These results correspond well to the TEM analysis results. See Figs. 9 and 10.
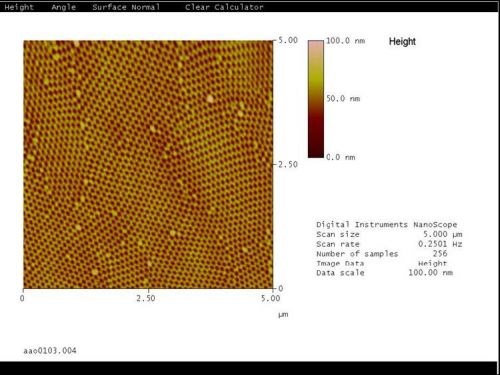
Figure 9 - AFM image of the top of an anodic oxide finish shows several columns per substrate grain arranged in a hexagonal close-packed nanoscale structural order.
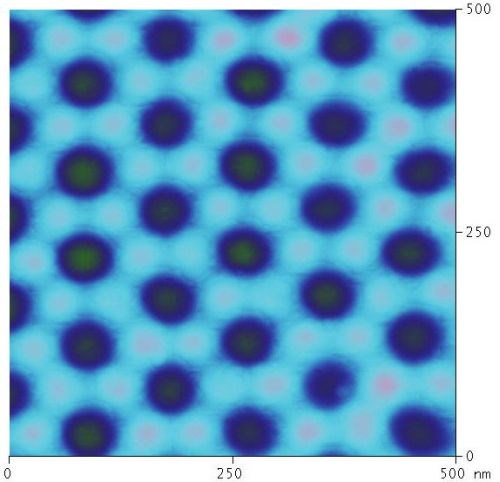
Figure 10 - AFM image of the aluminum substrate after the anodic oxide was chemically removed shows the arrangement of the oxide on the substrate. Each pore center is represented by a dark spot. The column walls are represented by the hexagonal arrangement of the light grey spots. The darker spots within the light grey hexagons are centers of impingement between column walls.
Images of the underside of the porous oxide finish show conclusively that each column base is rounded at the bottom and that the interstitial spaces are regular but can vary. This result corresponds to the TEM results that showed structural order of the anodic finish can be disrupted at the finish - substrate interface due to substrate defects that intersect the surface. Disruption of the structure therefore occurs between columns, at the knitlines, of the anodic oxide and clearly shows the importance the substrate quality has on the finish quality. In addition, these images clearly show no evidence of a continuous underlying barrier layer, but show that each column is part of a nanoscale finish network. See Fig. 11.
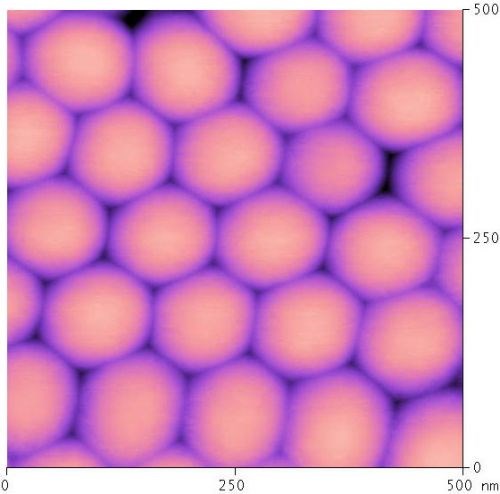
Figure 11 - AFM image of the underside of the anodic oxide finish after removal from the aluminum substrate. Note the simple hexagonal arrangement of each rounded column bottom. Also note the variations in the interstitial spacing. Each column measures approximately 100 nm from edge to edge.
Discussion
Intensive microstructural and chemical analyses of several types of anodized aluminum coatings were performed. The results provide increased insight regarding the mechanism for anodic film formation as well as its resultant chemistry. The analysis results consistently show that process modifications, whether chemical (to the electrolyte) or electrical (current density changes) impart distinct structural changes to the anodic oxide finish that are predictable and easy to control, and that the structure of the anodic oxide is of a nanometer scale.
By virtue of these changes and the predictable manner in which they can be achieved, we proposed the Constraint Concept of Porous Oxide Finish Formation. This theory explains how various oxide microstructural characteristics are achieved through initial surface reconstruction (corrosion of the substrate surface), through electric field effects during finish growth and through diffusion and mass transport that occur within the anodic oxide during anodizing, and how they change through modifications to the process.
Surface reconstruction
A comprehensive thermodynamic treatment of chemisorption, which precedes surface oxidation, is presented in Murr.12 It is important to note that the oxidative process of exfoliation on passive metals such as aluminum, will take place only in protonic acids which oxidize, for example, in sulfuric acid, chromic acid, oxalic acid, phosphoric acid, etc. Other acids will tend to corrode by other mechanisms, such as pitting. It is also important to note that throughout the anodization of aluminum and aluminum substrates, we are creating the thermodynamic conditions to make only hydrated aluminum oxide. Therefore alloy additions and other contaminants retard the reaction kinetics.
The “infant” anodic oxide finish begins as corrosion nucleation points over the entire substrate surface. On ideal surfaces, without alloy additions, atomic level defects or other surface contamination, nucleation will be ordered and the number of nuclei will be governed by the temperature of the anodization process. That is, lower temperature processes will exhibit fewer nuclei than higher temperature processes. As the exfoliation corrosion process continues to favor aluminum oxidation, the nuclei will grow outward, while consuming the substrate, to form a contiguous passive layer comprised of many ordered oxide flakes. The initial reconstructed ideal surface will have the appearance of a hexagonal bubble layer.
However, in industry we rarely encounter an ideal substrate. Nucleation sites will exhibit preferences for the more ideal such as certain crystallographic surface orientations, and other atomic level defects may impede nucleation, such as vacancies, dislocations, steps or ledges. Grain boundaries which can function as sinks for atomic level defects can also impede nucleation. Alloy additions which may be inert to the anodization process can retard oxidation kinetics uniformly, as when copper dissolves into the electrolyte while the aluminum oxidizes, or non-uniformly, as when inert inclusions such as silicides or insoluble alloy additions, such as lead, segregate and intersect the substrate surface. Our TEM analysis results clearly document these substrate - finish interactions, seen in Figs. 4 and 5.
An explanation for this is that in all types of aluminum anodization, the thermodynamics of the process seek to oxidize free aluminum. In alloy solutions, the kinetics will be retarded as the available aluminum is oxidized. The surface will be reconstructed, but more slowly than with a pure aluminum substrate. Depending on the size and extent of the substrate crystal defect, the reaction at the substrate will nucleate and renucleate as long as there are aluminum atoms available to oxidize. Therefore defect pockets of dislocations or vacancies will exhibit multiple nucleation points. Solute atoms within the aluminum lattice structure, such as copper, will be dissolved into the electrolyte as aluminum oxidation proceeds, and may redeposit onto the finish when oxidation kinetics no longer dominate the anodization reaction.
In segregated alloy solutions with inert inclusions, corrosion will proceed about and even around the inclusions, lifting them into the anodic oxide finish as it grows out from the substrate. The impact of inert or insoluble defects such as inclusions, grain boundaries and atomic level sinks on the finish is in the spacing of the nuclei. This disruption in order can lead to irregular growth and irregular intercolumn spacing. However, as the surface is consumed and a more ordered surface is presented for oxidation, the finish recovers as growth takes place, as in Fig. 6.
Finish growth
Without the imposition of an external electrostatic potential, the initial oxide layer would grow in excess of a monolayer to an equilibrium thickness we refer to as “native oxide” or a passive layer. Regardless, film growth requires diffusion of oxygen and the metal through the forming layer. Because the anodization reaction is one of simultaneous substrate consumption with film growth, we must consider the nature of the oxide network formed to understand how the passive layer can be developed to form the ordered microstructures familiarly recognized as anodic porous oxide coatings.
As surface reconstruction proceeds on the substrate surface, a certain amount of lateral finish growth occurs when the individual nuclei consume the aluminum, forming a monolayer. Preferential nuclei, formed at sites on the substrate surface where the energy required for adsorption was lowest, will continue to grow and develop as long as there is available free aluminum to oxidize, and the thermodynamic work function of the metal is such that the oxidation reaction is favorable. Work function can be defined as the difference in energy between the electrostatic potential outside the forming oxide layer and the electrochemical potential of the anode. Under atmospheric conditions, the oxidation reaction will cease as the passive layer exhibits continuity, causing the work function of the aluminum to reach equilibrium (equal zero), yet will begin again if the passive layer is scratched or otherwise disturbed, a characteristic referred to as “self-healing.”
With a deliberate increase in work function, such as through the introduction of a chemical contaminant to the atmosphere (as in corrosion) or through the imposition of an external current bias (as in anodizing) and/or a change in temperature (as in corrosion or anodizing), the passive network continues to grow. The infant nuclei become a network of hydrated, charged flakes that impinge on one another. As the reaction kinetics continue to favor the initial oxidation sites, exfoliation of the aluminum surface will proceed in a contaminated atmosphere. In anodizing, the impinging flakes repel one another and grow outward from the substrate surface, forming a unique network of nanoscale corrosion cells. Each cell exhibits the nucleation point at its rounded base; the flakes grow to become column walls which circumscribe the central pore. See Fig. 12.
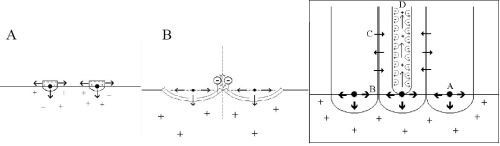
Figure 12 - “Constraint” concept of porous oxide finish formation:
A. Preferential nuclei form base of pore.
B. Repulsive forces between similarly charged oxide “flakes” foster outward growth and initiate pore formation.
C. Mass transport and diffusion across column walls form knitlines.
D. Repulsive forces within pore maintain the erect nature of the column and dynamic flow of the electrolyte.
The Ion Pump
The feature critical to the forming anodic finish is its central pore. As the base network of oxide flakes impinge on one another and grow outward, field effects created on the inside surface of the flakes repel each other such that the erect nature of the cellular network is maintained from the inside out. The pore wall becomes a dynamic surface as the oxidation reaction continues. As the electrolyte enters the porous structure, the anion, which is in part oxygen, reacts with aluminum ions diffusing through the finish at the pore surface. Hydrogen ions as H+ as well as hydrogen gas are ejected from the primary reaction sites and so even though reaction occurs due to adsorption of the counterion on the pore surface, the ionic activity and repulsive electric field forces keep the pore from building up and closing itself. The column walls increase in thickness due to oxide formation, and finish displacement is upward and away from the pore surface. Therefore, the source for the formation of ordered pores throughout the oxide structure is the result of electric field effects and ionic activity within the pore of each nanoscale corrosion cell of our finish, not dissolution by the electrolyte.
As the work function favors finish growth, the ion pump operates and maintains the central pore and outward finish growth. Consumption of the aluminum continues as aluminum ions diffuse to the pore surface, the cell walls get thicker and the oxide network builds. Transport occurs across between the individual cells knitting the structure together. The results of the TEM and AFM studies show that with an increase in current density of about 2×, the pore diameter increases by at least 2×. This result is supported by data published by Brace.13 As the work function approaches zero, when the product of the resistance of the finish and the current density equal the imposed external potential, the ion pump slows down and eventually stops. Hence, the activity of the ion pump determines the finish thickness.
Adsorption of less reactive (passive) species on the pore wall, diminish field effects and the columnar structure can no longer be maintained. Depending upon the robustness of the structure, the column walls won’t increase in thickness. They remain thin and collapse, forming the random structures of modified Type I or chromic acid anodic finish that automatically exhibit limited thickness. Therefore, the inert, stable nature of adsorbed complexes on the inside surfaces of the pores disables the “ion pump”. See Fig. 13.
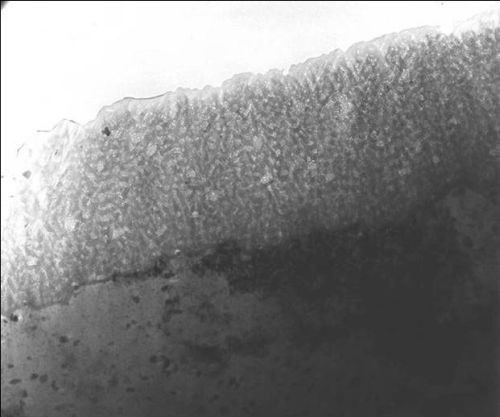
Figure 13 - TEM micrograph of a chromic acid (Type I) anodized finish. Microstructural change and finish thickness are altered by the adsorption of chromate ion on the pore surface.
When the work function of the anode approaches zero, the oxidation reaction no longer dominates and deposition of electroactive components within the electrolyte occurs at higher resistance portions of the anodic oxide finish if the substrate remains in the electrolyte. This explains the deposition of copper on the surface of the extended exposure samples determined through XPS analysis as well as the deposition of copper on anodized die castings that exhibit high levels of alloy segregation. These reactions occur because the pore wall is negatively charged, giving the entire finish a net negative charge, and it becomes an additional surface for adsorption of various electrolyte species.
If there is no additional flux due to ionic transport through the finish (i.e., if the ion pump is disabled), the pores become thicker and close themselves with adsorption, as described above. However, this characteristic of the pore also explains sealing phenomena. A certain amount of reductive dissolution of the finish occurs to enable dyeing, sealing and other post treatments of the anodic oxide through solution access at the pores and adsorption of surface network modifiers.
The formation of knitlines
Consideration must also be given to the outside surfaces of the column walls. As the oxide flakes impinge and grow outward, it is apparent that diffusion occurs across the column wall “knitting” the structure together. The stability and robustness of the final structure appear to hinge on this portion of the film formation. This is because there is no dynamic flux or ion flow on the outer walls of each column, as in the pores, which can disturb the formation of the final hydrated aluminum oxide species. Nevertheless, substrate surface conditions that disrupt surface reconstruction can disrupt knitline formation between each column. Therefore, the integrity of the finished film often is based on the integrity of the knitlines.
Summary
Through extensive analysis of anodic oxide finishes produced through a variety of parameters commonly utilized to form Chromic Acid (Type I), Commercial (Type II) and Hard (Type III) anodic oxide finishes on aluminum, the following conclusions can be drawn regarding the mechanism for film formation.
- The kinetics of anodic oxide film formation are governed by (1) the thermodynamics at the surface and (2) diffusion and mass transfer across the oxide layer as it forms.
- Surface reconstruction creates a network of nanoscale corrosion cells that, when the work function is greater than zero, become the anodic oxide finish.
- The columnar structure of the anodic finish is the result of lateral film growth and impingement of “infant oxide” flakes on one another. The repulsive forces of the similarly charged flakes foster outward growth.
- Pores are formed through repulsive field effects on the “inside” surfaces of the flakes, as they grow outward from the substrate.
- The rate of substrate oxidation initially exceeds the rate of adsorption of electrolyte network modifiers on the inside surface of the pores.
- A critical time exists where substrate oxidation ceases to be the dominant reaction and the work function of the reaction becomes zero.
- Film modification is facilitated by reactive species in the electrolyte that adsorb on the pore walls as the work function approaches zero.
- The integrity of intercolumn knitlines governs the integrity of the anodic film in service.
- The anodic oxide finish is of a nanoscale. Depending on anodizing conditions, the size, distribution and density of columns per grain substrate can be controlled.
Conclusion
Under typical atmospheric conditions, a native oxide or passive film naturally forms on aluminum. The native oxide layer is non-uniform, thin, and non-coherent. Nevertheless, the native oxide imparts a certain level of corrosion protection, provided the environment contains no unusual contaminants. Exfoliation, the formation of a network of oxide flakes or “leaves” on the aluminum surface is an example of how corrosion of the surface can be changed through the introduction of sulfur to the environment. In fact, removing sulfur from the atmosphere can control the exfoliation of aluminum.14-16
Anodizing can be viewed as the deliberate controlled exfoliation corrosion of the aluminum surface in oxidizing acids. By promoting exfoliation corrosion of the surface, a uniform, continuous protective oxide finish is formed. Its unique columnar structure has been extensively studied, yet the mechanism of porous oxide formation on aluminum substrates is not fully established. This paper proposes a mechanism for porous oxide formation based in corrosion science theory of surface reconstruction forming a nanoscale structural network of electrochemical cells. The theory is supported by results of comprehensive microstructural and chemical studies of conventionally anodized aluminum and aluminum alloy samples as well as samples anodized with modified processes.
A basic understanding of porous oxide formation through anodization enables many advantages for the industrial metal finisher. Two of the biggest advantages are: (1) the ability to control the type and therefore the properties of the anodic oxide finish (and that goes beyond simple Types I,
II and III anodizing processes!) and (2) the ability to enter other industries that to date, may have seemed unapproachable or inappropriate for our old, mature, unchanging process. So take heart and be energized…we were the first to provide a nanoscale finish!!
References
1. G.D. Bengough & J.M. Stuart, British patent (1923).
2. Caboni, Italian patent (1936).
3. J. Von Fraunhofer, Basic Metal Finishing, Chemical Publishing, New York, NY, 1976.
4. Clariant, Aluminum Finishing Class Notebook, 1991. http://sundoc.bibliothek.uni-halle.de/diss-online/04/04H055/t2.pdf, 2004 (last accessed 09/2020).
6. H.H. Wang, et al., Materials Research Society Symposium Proceedings, 775, 107 (2003).
7. M.A. Ryan & J.-P. Fleurial, Interface, 11 (2), 30 (2002).
8. J. Runge & A. Pomis, Proc. AESF SUR/FIN 2000 Technical Conference, AESF, Washington, DC, 2000; p. 267-278.
9. J. Runge A. Pomis, & Th. Nussbaum, Proc. Aluminium 2002 Conference - Essen, September, 2002.
10. J. Runge & A. Pomis, Proc. AESF Aerospace/Airline Plating & Metal Finishing Forum, AESF, Washington, DC, 2002.
11. J. Runge-Marchese & Th. Nussbaum, Proc. AESF SUR/FIN 1998 Technical Conference, AESF, Washington, DC, 1998; p. 531-540.
12. L. Murr, Interfacial Phenomena in Metals and Alloys, Addison-Wesley Publishing Co., Reading, MA, 1975.
13. A. Brace, The Technology of Anodizing Aluminium, 3rd Ed., Interall Srl, Modena, Italy, 2000.
14. H.H. Uhlig & R.W. Revie, Corrosion and Corrosion Control, John Wiley, New York, NY, 1985.
15. D.A. Jones, Principles and Prevention of Corrosion, MacMillan Publishing Company, New York, NY, 1992.
16. ASM International Metals Handbook, 10th Ed., Vol. 13, Corrosion, ASM International, Materials Park, OH, 1985.
About the author

Dr. Jude Mary Runge is the 2020 recipient of the NASF William Blum Scientific Achievement Award. Her career as a metallurgical engineer and surface finishing expert spans nearly 40 years in industrial, government and academic professional settings. Beginning in 1982 at Northrop Corporation, Defense Systems Division, the largest government contractor in the state of Illinois, and culminating as a metallurgical consultant and anodizing specialist in her own company, CompCote International, Inc. As well, she is a Consultant in Manufacturing Design as a Subject Matter Expert in Anodizing for Apple, Inc. Her analytical expertise and experience encompasses a variety of manufacturing processes for all materials as well as the science and engineering of many aspects of metal finishing. Although an accomplished metallurgical engineer in various fields, she is recognized internationally as a nonferrous specialist focusing on aluminum and aluminum alloy processes as well as anodizing and the theoretical treatment of porous oxide formation. Dr. Runge received her Ph.D. in metallurgy at the University of Illinois at Chicago under Herbert H. Uhlig Corrosion Scholar, Dr. Michael McNallan. She is the inventor and owner of the patented CompCote® process. She is recognized internationally as an author, lecturer and teacher. She is the author of The Metallurgy of Anodizing Aluminum, published by Springer Nature in April, 2018 and Division Editor to ASM Handbook, Volume 2A: Aluminum Science and Technology, published in December, 2018.
* Corresponding author:
Dr. Jude Mary Runge, President
CompCote International, Inc.
206 North York Road, Suite C
Elmhurst, Illinois 60126
Phone: (630) 617-3908 (US)
Fax: (630) 617-4209
E-mail: jude.runge@compcote.com
Related Content
Chicago-Based Anodizer Doubles Capacity, Enhancing Technology
Chicago Anodizing Company recently completed a major renovation, increasing its capacity for hardcoat anodizing and Type II anodizing.
Read MoreA Smooth Transition from One Anodizing Process to Another
Knowing when to switch from chromic acid anodizing to thin film sulfuric acid anodizing is important. Learn about why the change should be considered and the challenges in doing so.
Read MoreAnodizing for Bonding Applications in Aerospace
Anodizing for pre-prep bonding bridges the gap between metallic and composite worlds, as it provides a superior surface in many applications on aluminum components for bonding to these composites.
Read MoreBryan Leiker, MFACA, Discusses CARB Public Hearing Over Calif. Hex Chrome Ban
Bryan Leiker, executive director, Metal Finishing Association of California, offers a recap of a January 27, 2023, public hearing conducted by the California Air Resources Board prior to an impending ruling on a proposed ban of hexavalent chromium use for finishing operations in the state.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More







.jpg;maxWidth=300;quality=90)













