Metal processing, which includes cleaning, finishing (anodizing, electroplating, etc.) and forming, ultimately results in metal contaminants in the processing wastewaters. Heavy metals such as arsenic (As), chromium (Cr), copper (Cu), mercury (Hg), nickel (Ni), lead (Pb), zinc (Zn) and others are typically present in metal processing wastewaters and require removal prior to discharge due to their potentially negative health effects in the environment. Further treatment beyond metals-removal can produce water that can be reused, thus improving the sustainability of the manufacturing process. This article will provide a brief overview of compliance and sustainability considerations for the effective treatment of metal-bearing wastewaters.
Discharge Compliance Considerations
The presence of heavy metals in wastewater is problematic for discharge compliance as the metals may be toxic or otherwise harmful to the environment. Whether the discharge goes directly into a receiving body of water (river, stream, etc.) or indirectly via an industrial and/or municipal treatment plant, the ultimate goal is to protect the water quality of the receiving water.
Consequently, these metals must be removed in order to meet the discharge limits set out in the facility’s wastewater pretreatment permit. Failure to remove the metals can result in negative consequences such as fines, legal enforcement action, and bad publicity that can accompany such actions.
In addition to understanding the metals requiring removal and the target concentrations for compliance, it is important to understand sustainability considerations and other characteristics of the wastewater that can affect removal of the target metals.
Sustainability/Recycle Considerations
Recovering wastewater can lower the overall facility water usage and improve the facility sustainability profile. In order to effectively recycle metal-bearing wastewater, other typically non-regulated contaminants such as calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), chloride (Cl), nitrate (NO3), phosphate (PO4) and sulfate (SO4) must be removed in addition to the heavy metals.
The extent of treatment required, and consequently the technologies employed, are determined by the contaminants that must be removed and by the recovered water quality required for the process(es). For example, recovering water to use in a cooling tower may typically be less technology-intensive compared to water recovered for use in a critical cleaning application.
Wastewater Evaluation and Characterization
After considering discharge compliance and sustainability/recycle opportunities, the application must be evaluated, and the wastewater properly characterized to determine the most suitable technology. Evaluation and characterization involve an understanding of the generation of the wastewater, as well as the constituents present in the wastewater.
Vital generating process information includes the wastewater flowrate (average and peak), expected daily volume and how that flow and/or volume is distributed throughout a working day. This information must be understood to properly size a treatment system.
Wastewater characterization is typically accomplished by chemical and instrumental analysis to understand not only the target metal (and other contaminant) concentrations, but also related parameters such as pH, conductivity, hardness, and organic content as these parameters will affect the efficacy and efficiency of the selected treatment technology.
Treatment Strategies
There are a number of technologies that can be employed to accomplish metals-removal. Typical treatment strategies to meet discharge compliance or water recycling goals include conventional precipitation treatment, media treatment, and membrane treatment.
Conventional treatment uses chemical treatment to change the characteristics of the dissolved metals and precipitate them as solid particles. The solid particles are then removed via settling or a membrane filter, such as an ultrafilter (UF). The chemical treatment may also treat or neutralize other species that prevent easy precipitation of metals. Some examples include cyanide destruction, typically using alkaline chlorination, and hexavalent chromium reduction using reducing compounds such as sodium sulfite or sodium metabisulfite. Conventional treatment is typically used only for discharge compliance treatment, on average reducing metals to 0.5-1 part-per-million (ppm), as the treated water still contains a high level of total dissolved solids (TDS) and is not suitable for direct recovery and reuse.
Media treatment uses granular adsorptive media or ion exchange resin to remove the metal ions from the wastewater. The media is typically employed in flow-through pressure vessels. The media beds are susceptible to solids fouling, thus adequate prefiltration must be employed ahead of the media beds. Ion exchange resins are regenerable, either offsite or onsite, while adsorptive media are single-use when used for metals-removal. Media treatment, which can reduce contaminant levels to single-digit part-per-billion (ppb) levels or lower, can be used for either discharge compliance or water recovery and reuse applications as the contaminants are removed from the wastewater and adsorbed to the media, thus producing a clean water stream.
Membrane treatment uses polymeric membranes to separate particulate and/or ionic species from the wastewater using pressure. Microfiltration (MF) filters down to 0.1-0.2 microns (µm), ultrafiltration (UF) to 0.005-0.1 µm and reverse osmosis (RO) to approximately 0.0001 µm. MF and UF are good for particle and colloid removal while RO will separate ionic species. Membrane processes separate; that is, they produce a clean water stream (permeate) and a more concentrated stream (concentrate) that still may require treatment for metals. MF and UF are typically used for discharge compliance applications as, similar to conventional treatment, the clean water stream is usually high in TDS. RO, on the other hand, is typically used for recovery and reuse applications as the tighter RO membrane can reject contaminants on the ionic level producing recovered water with low-ppm or ppb levels of contaminants.
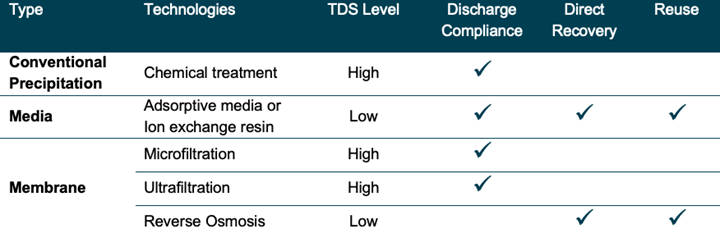
Comparison chart of technologies and applications.
Photo Credit: Evoqua
Wastewater Application Examples
To illustrate specific wastewater treatment (WWT) methods that can be implemented to achieve compliance and sustainability goals within the metal finishing industry, the following examples will be explored.
When a POTW Lowers Discharge Limits
Conventional metal hydroxide WWT methods are commonly used to meet categorical discharge limits for metals with targets down to 0.5-1 mg/L. When a POTW implemented a lower 0.09 ppm cadmium (Cd) limit for an aerospace component manufacturer, an effective method for further metal reduction was needed to prevent permit violations and fines for exceedances. Treatability studies were performed on WWT effluent samples to characterize the Cd and other constituents and identified ion exchange (IX) as the most effective method of selectively polishing residual dissolved Cd to < 1 ppb.
The wastewater ion exchange (WWIX) system included bag prefiltration to remove suspended solids (TSS), cartridge filtration to remove sub-micron Cd particles, and two leased exchangeable IX vessels with a proprietary heavy-metal selective adsorbent media. The adsorptive media reduced the Cd and other regulated heavy metals to single-digit ppb concentrations while allowing non-regulated constituents to pass.
This solution allowed the customer to continue operating the existing WWT system to remove the bulk of the process metals, while quickly installing the WWIX system as a final polishing step to achieve compliance. In addition, to maintain discharge compliance, the spent media was also able to go through a recovery process allowing the generator to implement a “green” approach by reducing overall liability and eliminating hazardous landfill waste.

Wastewater ion exchange tanks for final metal polishing.
Precipitating a Discharge Solution
Many facilities operate WWT systems that have been around for 20 years or more. One such facility, a large manufacturer of aeronautical and space components, was operating an older hydroxide precipitation system to treat their circuit board manufacturing wastewaters for the removal of copper, lead, nickel, silver, and zinc. The age of the system resulted in decreased metals-removal efficiency and increased chemical usage and maintenance and precipitated the need for system replacement.
An evaluation of the application confirmed that hydroxide precipitation was the technology of choice. To optimize the footprint, a 50 gallon-per-minute (gpm) continuous precipitation system (CPS) was selected; the CPS incorporated the reaction tanks, flocculation, and clarification chambers in one integrated, skid-mounted unit with sludge dewatering handled by a separate filter press. Use of the CPS resulted in the facility maintaining discharge compliance with an improved WWT system in a smaller footprint, compared to separate unit operations.

Continuous precipitation system for conventional metals treatment.
Bringing Water Recovery and Quality Goals in for a 3-Point Landing
As companies have been placing increased emphasis on sustainability efforts internally and from end-users and suppliers, introducing effective water recovery methods that reduce water consumption, maintain stringent water quality standards, and meet discharge limits can be critical for achieving operational and environmental objectives.
A manufacturer of large military and commercial aircraft structures, facing increased scrutiny from the local POTW due to permit violations, became determined to reduce the volume of treated water being discharged to the POTW. This more sustainable approach would reduce incoming water costs by returning treated wastewater to the rinse tanks and would also improve final rinse water quality to reduce rejected and re-worked parts.
Operational process data was reviewed, samples were evaluated, and treatability studies were performed to determine the optimal treatment system design. Ion exchange was selected to both remove hexavalent chromium from the conversion coating rinses and lower the conductivity in the treated water to allow its reuse.
After initially piloting a 10-gpm WWIX system in rental service vessels to demonstrate the effectiveness of the system design, the facility implemented a full-scale, 150-gpm rinse recycling system consisting of both cation and anion WWIX service vessels treating the chromium-bearing rinses. The treatment and recovery approach ultimately allowed the facility to achieve its treatment and recovery objectives quickly.
“Zincing” About Water Recovery
A metal component manufacturer operated an aged metals precipitation system used for treating their metal-bearing wastewaters, which mainly consisted of wastewaters from zinc phosphating operations. This labor-intensive WWT system was hydraulically limited and was not able to keep up with the evolving facility water treatment needs. In addition to evaluating a less labor-intensive WWT system, the facility wanted to investigate water recovery.
Thorough waste stream characterization and treatability testing confirmed that metals precipitation was the preferred treatment technology for this application. A microfiltration (MF) membrane system was selected to replace the old gravity settling system for separation of the precipitated solids. The product, or permeate, from the microfilter also was an ideal feed for an RO membrane system to recover a portion of the treated wastewater.

Reverse osmosis system for water recovery.
On-site pilot testing of a combined MF/RO treatment system proved the efficacy of the treatment combination resulting in the installation of a 65gpm MF system followed by an RO system designed to recover 60% or more of the MF permeate. The full-scale system also utilized the existing chemical pretreatment equipment on site with some minor modifications. Ultimately, the wastewater treatment goals of a reliable, semi-automatic metals-removal system were met with the added benefit of the recovery of a majority of the treated water.
Conclusion
Compliance with ever-tightening wastewater discharge limitations, meeting sustainability and water recovery goals, and manufacturing finished products with demanding quality specifications are all part of today’s metal processing operations. Choosing the right mix of treatment technologies depends on proper waste stream evaluation and characterization, as well as clearly established treatment goals. Add that to partnering with an appropriate water technology company with a range of treatment technologies will ensure success in achieving wastewater treatment goals.
About the Authors

Chris Riley
Chris Riley is the Technical Services Director for Evoqua’s WWIX business and oversees technical support activities for customers as well media regeneration and quality control. Chris has been with Evoqua for over 26 years, holds a bachelor’s degree in Chemical Engineering from Michigan Technological University, a Master’s degree in Civil (Environmental) Engineering from the University of Minnesota, and is a registered professional engineer.

Mark Korzenecki
Mark Korzenecki is a Business Development Manager at Evoqua with over 17 years of experience in WWIX, adsorptive media and wastewater treatment systems for the removal of inorganic/heavy metal contaminants from wastewater. Prior to joining Evoqua, he held account management roles in the hazardous waste/environmental services industry.
Related Content
Understanding and Managing White Spots on Anodized Aluminum
Having trouble with spotting defects when anodizing? Taj Patel of Techevon LLC offers a helpful overview of the various causes of white spots and potential solutions.
Read MoreCalculating the Cost of Powder Coating
How can you calculate the cost of powder coating a component if you only know its surface area? Powder coating expert Rodger Talbert has the answer.
Read MoreAn Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreZinc Phosphate: Questions and Answers
Our experts share specific questions about zinc phosphate and pretreatment
Read MoreRead Next
Zirconium Pretreatment Offers Simplicity and Performance
Pretreatment supplier explores industrial, agricultural markets for phosphate-free solution.
Read MoreNASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 3rd Quarterly Report
The NASF-AESF Foundation Research Board has selected a project on addressing the problem of PFAS and related chemicals in plating wastewater streams, studying PFAS destruction via electrooxidation and electrocoagulation. This third quarter report continues the work on electrocoagulation, characterizing the floc morphology generated from plating wastewater, varying conditions in the electro-oxidation solution.
Read More




















