A New Sacrificial Corrosion Protection Mechanism for High Performance Zinc/Aluminum Flake Coating Systems and Applications
This report describes how the corrosion-protection capability of a new zinc/aluminum flake coating system is supported by a sacrificial corrosion-protection mechanism.
By Shusaku “Shu” Ishikawa and Yasuharu Takayama, Corrosion Specialists, Surface Treatment Group, Engineering, Yuken Industry Co. Ltd., Japan
Editor's Note: This paper is a peer-reviewed and edited version of a presentation delivered at NASF Sur/Fin 2013 in Rosemont, Ill., on June 12, 2013. A printable PDF version is available by clicking HERE.
ABSTRACT
Zinc/aluminum flake coating systems used for the automotive industry must have a thin film with high corrosion protection performance to meet the demanding requirements. Numerous steps are required to achieve a high level of corrosion resistance, and therefore, it is essential to reduce manufacturing costs and increase productivity. A new zinc/aluminum flake coating system has recently been developed. The coating creates a robust and versatile thin film with uniform covering power, and it can achieve high corrosion protection while not requiring the conventional full course of dip/spin process steps. This report, using some application examples, describes how the corrosion protection capability of this system is supported by a sacrificial corrosion protection mechanism.
Keywords: Zinc/aluminum flake coatings; corrosion protection.
Introduction
In wide use like zinc and zinc alloy plating, zinc/aluminum flake coatings are often selected for the surface treatment of steel parts in the automotive and construction industries. Among the parts manufactured in those industries, the surface treatment for small parts or fasteners requires a thin film because of the requirements of dimensional accuracy. In particular, as parts processed with zinc/aluminum flake coatings quite often need to satisfy stringent requirements for corrosion resistance, the key is to design a thin film in such a way that good corrosion resistance can be sustained with the thin film. Therefore, it is essential for us to understand better the corrosion resistance mechanism of the coating film.
There have been many studies on the corrosion resistance mechanism of zinc alloy plating systems requiring high corrosion protection. It has been widely accepted as a given that it is important to form a corrosion product or basic zinc chloride which works as an insulator, instead of conductive zinc oxide, in an environment where chlorine ions are present. On the other hand, there have not been many studies on the corrosion protection mechanism of the zinc/aluminum flake coating film.
Zinc sacrificial corrosion protection is the main corrosion resistance mechanism of the zinc/aluminum flake coating film. Therefore, in order to achieve high corrosion resistance, we can easily imagine that the corrosion product developed from the film plays an important role, just as with zinc alloy plating.
In this study, we examined how the coating film develops a corrosion product in a corrosive environment containing chlorine ions, and looked into the correlation with corrosion resistance.
Experimental
Preparation of test samples
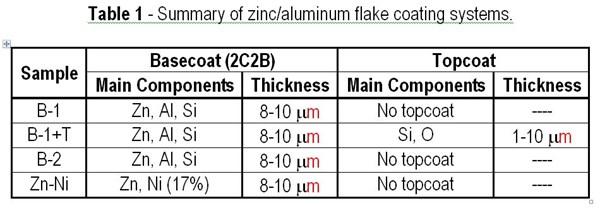
We used two types of basecoats with different coating components in each of the test samples, designated B-1 and B-2. B-1 is our original basecoat system, which can provide high corrosion protection when it is used with our topcoat system applied on top. B-2 is a new basecoat system developed to improve the corrosion resistance of B-1. In addition to B-1 and B-2, we used a combination of B-1 and the topcoat (B-1+T). Zinc nickel plating (Zn-Ni) was also included as a comparative material. Test coupons made of cold rolled steel (JIS-G3141) were first coated with each coating system using a bar coater. The basecoat was cured at 260 to 300°C, while the topcoat was dried at 100°C when it was applied over B-1. The processed test coupons were then cooled down to room temperature and left sitting for more than 8 hr before they were used for the test. The zinc-nickel plating we used for comparison was not passivated. Table 1 shows a summary of each test film.
Corrosion protection evaluation
For an accelerated corrosion test, we performed a cyclic corrosion test (CCT) with one cycle composed of a salt spray (5% NaCl, 50°C) for 17 hr; dry (70°C for 3 hr; salt spray (5% NaCl, 50°C) for 2 hr; and natural dry (25°C) for 2 hr. Some of the test coupons were put into a salt spray test (SST)(5% NaCl, 35°C) and a salt water immersion test (5% NaCl, 25°C, exposed to the atmosphere).
The corrosion protection performance was evaluated at the number of cycles or the number of hours when red rust was seen to occur on the test coupon.
Corrosion product evaluation
X-ray diffractometry (XRD) was used to identify corrosion products. The details of the XRD are:
Equipment:
Horizontal Type X-Ray Diffractometer PW3050 manufactured by Phillips
Measurement conditions:
- Copper anticathode
- X-Ray tube voltage: 40kV
- X-Ray tube current: 30mA
- Scan axis: 20θ
- 2θ: 10°- 90°
- Scan step: 0.02°
- Step time: 0.5 sec/step
Surface condition evaluation
We used a SEM-EDS to analyze the film surfaces and corrosion products before and after the corrosion resistance evaluation. The details of the SEM-EDS are:
- Tester: JSM-6480A (JEOL Technics Ltd.)
- Accelerating Voltage: 15kV
- Magnification: x2000
Time-dependent changes in natural potentials
Natural potentials were measured in a 5% NaCl solution (exposed to a 25°C atmosphere) as a method to evaluate the films electrochemically. A silver / silver chloride electrode in saturated potassium chloride was used as a reference electrode. The measurements were taken over 10 days.
Results and discussion
Corrosion resistance evaluation results
Corrosion resistance evaluation results of the test coupons from the SST and CCT respectively, are shown in Figs. 1 and 2. When we look at the zinc/aluminum flake coating films only, B-1 (original basecoat only) failed first both in SST and CCT, followed by B-1+T. B-2 (new basecoat without topcoat) performed the best. As expected, B-1 with the topcoat had a better corrosion resistance performance than that of B-1 only. In addition, B-2, the improved version of B-1, was able to achieve a high corrosion resistance without the topcoat.
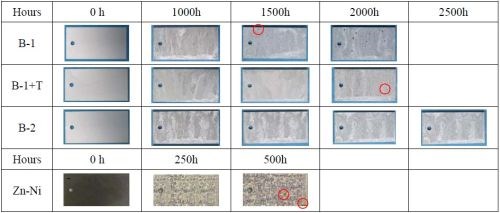
Figure 1 - SST results.
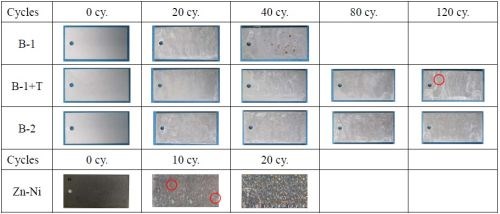
Figure 2 - CCT results.
Corrosion product formation process
Figure 3 shows the XRD results for each test coupon after a 24-hr salt water immersion test. All the films displayed a peak around 2θ = 11° after the 24-hr salt water immersion. These peaks indicate the presence of basic zinc chloride. We confirmed that the B-1 and B-2 films and the Zn-Ni plating film had already developed basic zinc chloride during the initial period. When B-1 and B-2 were compared, the peak showing zinc (2θ = 43°) was more reduced in B-2. On the other hand, the B-1+T film barely indicated a peak of basic zinc chloride, and as well the XRD pattern hardly changed before and after the immersion. We assume that this is because the topcoat-controlled time-dependent fluctuations by blocking off corrosive elements. No film was verified to have a conductive zinc oxide peak (2θ = 61°, 73°).
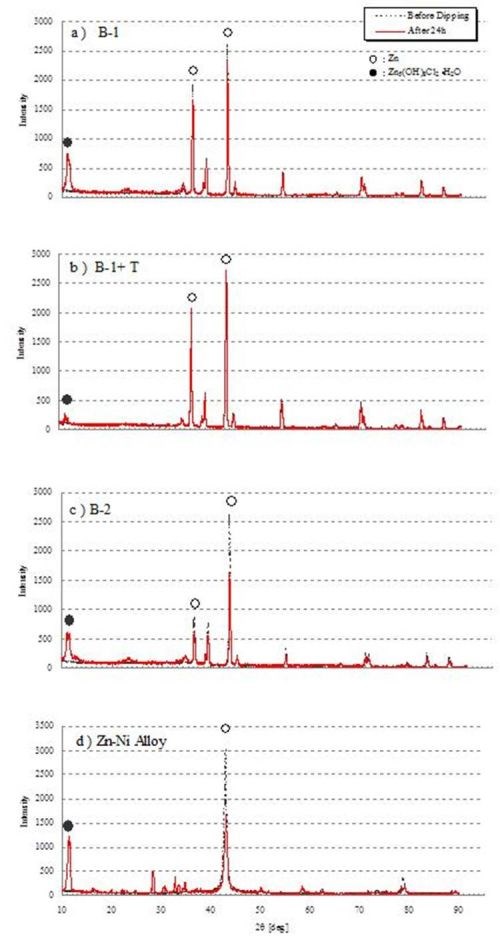
Figure 3 - XRD pattern of each film before and after the 24-hr NaCl immersion test:
Black dotted line: Status before salt water immersion test.
Red solid line: Status after 24-hr immersion test.
Figure 4 shows the XRD intensity change in each test coupon at times up to 720 hr of salt water immersion. The first graph indicates XRD intensity changes of basic chloride zinc and basic zinc carbonate, while the second graph shows the zinc XRD intensity change in each film. Both B-1 and B-1+T showed that the intensity of basic zinc chloride increased as time progressed, while B-2 showed a rapid increase in basic zinc chloride during the initial period (24 to 72 hr). The Zn-Ni plating film formed basic zinc chloride during the initial period, but it changed to basic zinc carbonate after 72 hr of the immersion test. The zinc intensity of the zinc/aluminum flake coating films decreased in proportion to the intensity of basic zinc chloride. On the other hand, the intensity change of the corrosion product did not necessarily match the zinc intensity reduction in the Zn-Ni plating film.
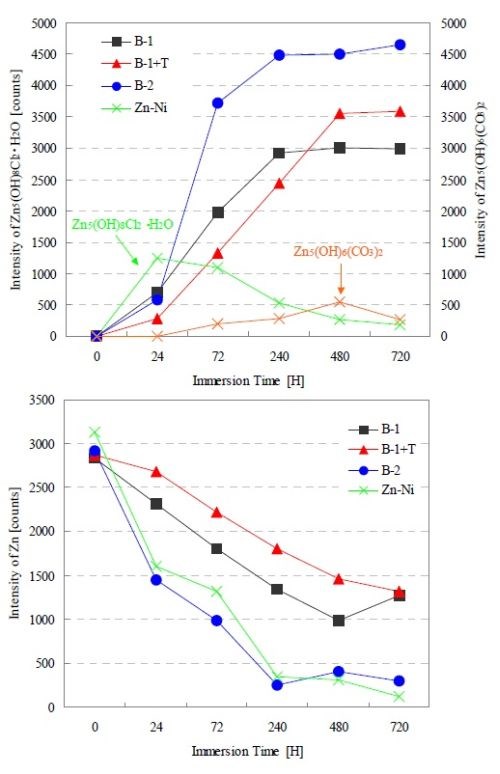
Figure 4 - XRD intensity changes of corrosion product and zinc in conjunction with the progression of the NaCl immersion test.
Figure 5 shows the XRD intensity change of each test coupon up to CCT 40 cycles and SST 2000 hr. We confirmed that the trends were very similar to those in the salt water immersion test. Although there were no changes in the corrosion products as a result of changes in the corrosive environment, we verified that the basic zinc chloride gradually decreased in the B-1 and B-1+T films as time progressed. The generation of basic zinc chloride in B-1+T was more gradual than in B-1. We believe this is because the topcoat blocked off corrosive elements, deterring time-dependent changes. As a result, the corrosion resistance performance improved while zinc corrosion was delayed. On the other hand, the B-2 film which demonstrated the best corrosion resistance developed basic zinc chloride during the initial stage, in contrast to the B-1+T film. We surmise that the B-2 film was able to achieve stable corrosion resistance as the film was covered by the corrosion product.
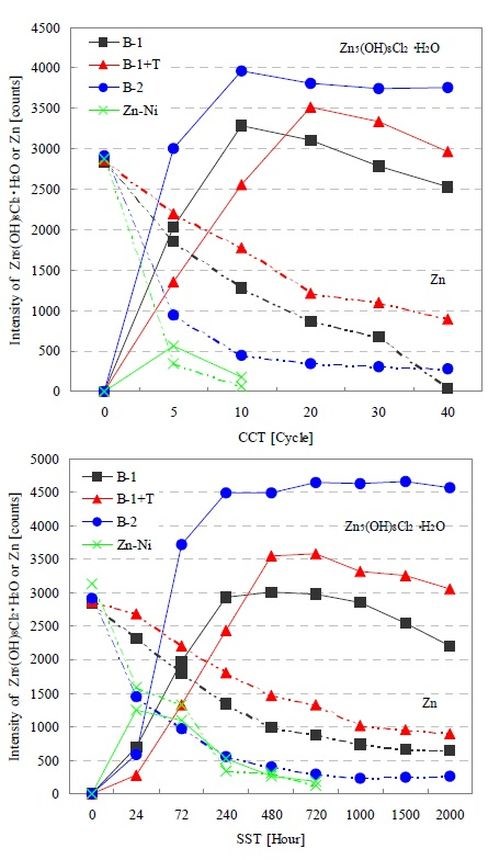
Figure 5 - XRD intensity changes of corrosion product and zinc in conjunction with the progression of CCT and SST.
Figure 6 shows the surface appearance of each film after 240 hr in SST. According to the results in Fig. 5, basic zinc chloride was identified in all the films. However, the surface appearance of each film varied. We saw granular basic zinc chloride in the B-1 film, and the granule rate decreased in B1+T and further declined in B-2. When the findings of Figs. 1, 2 and 5 are examined, we can point out that the basic zinc chloride comprised of finer crystals was more stable than that comprised of larger ones in the corrosive environment, demonstrating greater corrosion resistance.

Figure 6 - Surface appearance of each film after 240-hr SST.
Time-dependent changes in natural potentials
Figure 7 displays the time-dependent change in each film immersed in a 5% NaCl solution. B-1, B-1+T and B-2 have a similar potential behavior, but B-2 shows the potential increasing slightly faster than the other two films. Based on this finding combined with the XRD results, we can point out that the potential of the B-2 film increased more quickly because it formed basic zinc chloride during the initial period. One fact commonly found in all of the zinc/aluminum flake coating films is that the potentials were around the same level as zinc dissolved during the initial period, that they then shifted to the noble direction after a 72-hr immersion, and that they became stable around -0.6 V. We assume that this is because the potentials became stable and protective of the surfaces, as a result of the formation of basic zinc chloride.
On the other hand, the natural potential of the Zn-Ni plating film gradually shifted to the noble side as time progressed, and became stable around -0.6 V. For a natural potential trend comparison, also shown in Fig. 7, we added zinc plating (without passivation) data. The potential of the zinc plating film was kept low while zinc was dissolving, but once the film dissolved, the potential suddenly increased. We believe that the aluminum in the Zn/Al flake coating films was controlling excessive zinc dissolution. We could not identify a clear correlation between the measured natural potentials and the corrosion resistance, but we did verify that the natural potential of a film needs to be stable around the natural potential of Fe (-0.4 to -0.5 V) to achieve high corrosion resistance.

Figure 7 - Time-dependent change in the natural potential of each film.
Summary
After we examined the relationship between the corrosion products generated on Zn/Al flake coating films during the corrosion acceleration tests and the corrosion resistance of each film, we confirmed the following:
- Zn/Al flake coating films can achieve high corrosion resistance by forming and maintaining basic zinc chloride as a corrosion product in a corrosive environment with chlorine ions.
- A film covered with basic zinc chloride during the initial stage can achieve superior corrosion resistance.
- The basic zinc chloride that develops on the film affects the corrosion resistance of the film, and basic zinc chloride, comprised of fine crystals rather than coarse granules, shows stronger corrosion protection.
- According to the time-dependent changes in the natural potentials we observed, it is important for a film to control excessive zinc dissolution and to keep its potential near that of iron to obtain high corrosion resistance.
Based on the study we conducted at this time, we were able to confirm that the formation of basic zinc chloride in a corrosive environment has a strong relationship with high corrosion resistance performance of Zn/Al flake coating films, just as is the case with zinc alloy plating films.
One anti-corrosion approach to achieve excellent corrosion resistance is the use of a topcoat which blocks off corrosive elements, delaying zinc corrosion. Another is to develop basic zinc chloride during the initial stage and use the corrosion product to block off corrosive elements, which we verified with the B-2 film presented here.
In the near future, we would like to investigate further what type of corrosion products are developed as influenced by film environments and structures that generate basic zinc chloride.
Further Reading
1. A. Sakoda, et al., “Relationship between Zinc Plating Corrosion Resistance and Corrosion Product, Surface Treatment Technology, 40 (1), 164 (1989).
2. A. Komatsu, et al., “Corrosion Resistance and Corrosion Protection Mechanism for Steel Plates Plated with Zn-Al-Mg Alloy in Accelerated Corrosive Environment,” Tetsu-to-Hagane, 86 (8), 2000.
3. H. Fukuzawa, “Zinc Corrosion and Corrosion Product,” Corrosion Protection Control, 2008 (2).
4. H. Hamada and T. Deguchi, “Formation Mechanism of Zinc Corrosion Products,” Corrosion Protection Control, 38 (12), 453 (1994).
5. T. Hashimoto, “Zinc and Zinc Alloy Plating Film Structure and Corrosion Resistance,” Surface Science, 22 (2001).
6. S. Fujita, “Automotive Steel Parts Evaluation and Application Technology (Corrosion), JFE Technology Newsletter No.4.
7. K. Hayashi and S. Tsujikawa, “Revision of pCl-pH Diagram of Zn-Cl-H2O System,” Zairyo-to-kankyo, 50, 292 (2001).
8. H. Obata, et al., “Study of Development Analysis and Evaluation of the High-Purity Metallic Material.”
9. H. Nagata, M. Matsunaga and K. Hosokawa, “Zinc-silicate Formation in Galvanized Steel, Pipes for Water Service and Their Relationship to Morphology of Corrosion Products, Zairyo-to-kankyo, 41, 816 (1992).
10. K. Aramaki, “Actions and Applications of Corrosion Inhibitors.”
Related Content
Trivalent Chrome Overview
As the finishing industry begins to move away from the use of hexavalent chromium to trivalent chromium, what factors should finishers consider as they make new investments? Mark Schario, chief technology officer for Columbia Chemical offers a helpful overview of this complicated topic.
Read MoreHow to Maximize Nickel Plating Performance
The advantages of boric acid-free nickel plating include allowing manufacturers who utilize nickel plating to keep up the ever-changing regulatory policies and support sustainability efforts.
Read MoreTroubleshooting Alkaline Zinc
One of the most common problems that can arise when plating with alkaline zinc is an imbalance of brightener in the solution. In this helpful Ask the Expert article, Chad Murphy of Columbia Chemical discusses how different zinc metal concentrations and brightener concentrations can impact efficiency.
Read MoreAn Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreRead Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More
















.jpg;maxWidth=300;quality=90)











