Acid Recycling at Plating for Electronics
Saving money, streamlining production, reducing waste and improving worker safety were some of the goals Plating for Electronics aimed for when it started investigating diffusion dialysis...
Diffusion dialysis is used to recover and recycle a range of industrial acids and acid mixtures. Successful applications include hydrochloric acid pickles, nitric acid rack stripping solutions, nitric and hydrofluoric acid etch solutions, methane sulfonic acid predips, fluoboric acid, phosphoric acid solutions and sulfuric acid anodize solutions.
At Plating for Electronics (PFE, Waltham, MA) spent hydrochloric acid solutions generated from pickling operations contributed to the hazardous waste stream. Engineers at PFE investigated hydrochloric acid recycling as a way of improving worker safety as well as reducing chloride levels in the treated plant effluent. The finishing job shop also was looking to reduce costs and toxic emissions and improve production quality.
At PFE, proposals for capital projects, such as implementation of diffusion dialysis, are required to undergo an environmental impact review and a review of the impact on manufacturing/operations. The project was required to help in the following areas.
- Streamline production and assure quality;
- Address the goals of waste reduction and better ensure worker safety; and
-
Determine merit by calculating internal rate of return.
What is Diffusion Dialysis?
Diffusion dialysis is a membrane separation process for recovering acids from dissolved metal-bearing solutions. Diffusion is the spontaneous movement of a material from an area of higher concentration to an area of lower concentration. Driven by the concentration difference, materials continue to move until the concentration difference no longer exists. Dialysis separates the molecules using the difference in the rate of movement of the molecules through a semi-permeable membrane.
When recovering acids this way, an anion exchange membrane acts as the semi-permeable barrier. It is placed between flowing water and acid streams containing dissolved metals. The anion exchange membrane has fixed positive charges on its surface. These positively charged locations attract the negatively charged anions in the solutions.
With hydrochloric acid baths, the predominant anion is the chloride ion. As the chloride ions are attracted to the membrane, they are also driven by the concentration difference to diffuse across the membrane to the water side. Simultaneously, the Thermodynamic Law of Electroneutrality (in solution, the total charge must balance) requires the transference of every chloride ion be accompanied by the transference of one positive charge. Positively charged ions, such as Fe+2 or other metals, are strongly inhibited from crossing the positively charged membrane because of repulsion between like charges. The hydrogen and H3O+ ions present in the acid solution are also positively charged but are able to cross the membrane with little hindrance. This is due to the high concentration of hydrogen ion in the acid solution and, in part, because of the highly associated nature of water that allows the hydrogen ion to effectively delocalize its charge. The net effect is that the rate of diffusion of hydrochloric acid across the membrane is an order of magnitude greater than that of the dissolved iron.
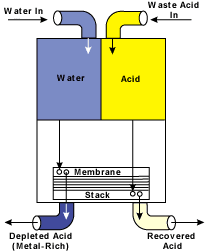
The water entering the dialysis system exits as a metal-depleted acid solution, and the acid solution entering the dialysis system exits as an acid-depleted dissolved metal-bearing solution.
Applied Diffusion Dialysis
The standard processing rate for diffusion dialysis systems is 1 liter/hr/sq meter (approximately 0.025 gal/hr/sq ft) of available anion exchange membrane surface area. To obtain the membrane area needed to process large volumes, the membranes are stacked between gasketed hydraulic flow spacers. These membrane stacks are usually standardized over a range of differing processing capacities.
Figure 1 illustrates a typical automated acid-recycling configuration. Once the acid and water are delivered to the recycling system, these streams then flow by gravity into the membrane stack (see Figure 2).
Once within the membrane stack, the acid diffuses into the water. The majority of the metal contaminants are left behind. Two streams are produced from the system. One is a purified recovered acid. The other is a metal-bearing, acid-depleted stream. The recovered acid is recycled back into the operating bath, and the metal-rich, acid-depleted stream is directed to waste treatment or a recovery/volume reducing system.
Implementing Acid Recycling
PFE installed a 5-gpd diffusion dialysis system on a 150-gal hydrochloric acid tank used for pickling. This bath was dumped once a month. The sizing of the dialysis system was based on the volume of spent acid previously produced, production rate and the efficiency of the dialysis process to recover the acid solution and remove the metal contamination. A "rule of thumb" requires that, at a minimum, the volume of spent acid that was previously discarded be recycled once through the dialysis unit over the same period of time it took to generate the spent acid.
The 5-gpd acid recycling system was installed directly on the process tank. Prior to acid recycling, the hydrochloric acid tank was dumped about once per month. After the unit was installed, the bath was not dumped for several years. However, due to increased production, the bath is dumped every 4-6 months. Additions of 4-8 gal of virgin acid are made each week to replenish depleted volumes due to dragout, exhaust and minor amounts lost in the dialysis process.
The following benefits were realized by using diffusion dialysis.
- Eliminated disposal costs and reduced inventory purchases estimated at $2,500 annually;
- Eliminated production downtime associated with dumping and recharging acid baths;
- Minimized direct operator contact with dangerous chemicals;
- Allowed for semi- automatic operation with minimal oper ating costs; and
- Improved process control, which improved quality and minimized waste.
Diffusion dialysis for acid recycling reduces acid purchases and eliminates or lessens neutralization or hazardous waste hauling costs and the related liability. Toxic chemical use is reduced, and the required reporting and handling of hazardous material and associated labor is greatly reduced. Consistent bath strength yields greater product uniformity and better quality. PFE found that dialysis can dramatically improve a facility's production quality and economic performance.
Related Content
Finishing High Reliability, Function Critical Parts
From safety critical automotive and aerospace components to lifesaving medical micro-components and implantable devices, Indiana-based Electro-Spec finishes applications that require zero failure rates.
Read MoreChecon Expands Market Offering with Acquisition of Umicore Electrical Materials USA, Inc.
The company is strategically working to meet future growth opportunities surrounding the use of precious metals and conductive materials like copper and aluminum.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More












.jpg;maxWidth=300;quality=90)








