Characterization of Electroless Deposition of Ni-P-Al2O3 and Ni-P-TiO2 from Alkaline Hypophosphite Baths in the Presence of Gluconate as a Complexing Agent
Electroless deposition of Ni-P-Al2O3 and Ni-P-TiO2, in the presence of gluconate as a complexing agent, on copper substrates was studied. The dependence of the composite formation on different plating variables was investigated. The deposited coatings were characterized using different techniques, including energy dispersive x-ray spectroscopy (EDX), x-ray diffraction (XRD), scanning electron microscopy (SEM), hardness and corrosion resistance testing. The amount of particles incorporated in the coatings increased with increasing particle concentration in the bath. However, the particle size greatly affected the amount incorporated. The addition of these particles changed the microstructure of the Ni-matrix, and enhanced the hardness and corrosion resistance.
by M.S. Ali Eltoum,1*A.M. Baraka,2 S.A. Abdel. Gawad2 and Elfatih. A. Hassan1
1Scientific Laboratories Department, Faculty of Science, Sudan University of Science &Technology, Khartoum, Sudan
2Chemistry Department, Faculty of Science, Cairo University, Cairo, Egypt
Electroless deposition of Ni-P-Al2O3 and Ni-P-TiO2, in the presence of gluconate as a complexing agent, on copper substrates was studied. The dependence of the composite formation on different plating variables was investigated. The deposited coatings were characterized using different techniques, including energy dispersive x-ray spectroscopy (EDX), x-ray diffraction (XRD), scanning electron microscopy (SEM), hardness and corrosion resistance testing. The amount of particles incorporated in the coatings increased with increasing particle concentration in the bath. However, the particle size greatly affected the amount incorporated. The addition of these particles changed the microstructure of the Ni-matrix, and enhanced the hardness and corrosion resistance.
Keywords: complexing agent, electroless deposition, copper substrate, matrix composite, corrosion resistance, polarization curves.
The structure of as-deposited electroless Ni-P is amorphous with high phosphorus content. However, this amorphous structure is metastable and undergoes a crystalline transition with increasing temperature. After adequate heat treatment, the coating becomes crystalline and its hardness and wear resistance are greatly improved.5
Embedding particles in electroless deposited metals is a convenient method of preparing composite coatings, and the particles increase the mechanical and physical properties of the coating.6 The activation energy of crystallization is lowered due to the presence of co-deposited particles in composite coating.7 The effect of particle size has been studied.8 Greater particle incorporation and uniform distribution was found in composite coatings obtained with 1.0 µm than with 50 nm or 0.3 µm particle sizes.
Using a complexing agent such as citrate in electroless Ni-P such as citrate,9 propylene glycol and urea10 is found to induce stability during plating. A survey of the literature shows that gluconate electrolytes have been used to electroplate metals such as nickel,11 copper,12 tin13 and zinc.14 No literature reference was found on the use of gluconate in electroless nickel plating.
The objective of the present study is to obtain Ni-P-Al2O3 and Ni-P-TiO2 composites from alkaline gluconate baths, studying the dependence of coating characteristics on several electroless plating variables. Our work also characterizes the coatings by using different analytical techniques such as SEM, EDX and XRD and examines the coating hardness and corrosion resistance.
Bath A | Bath B | Bath C | |
Nickel sulfate | 25 g/L | 25 g/L | 25 g/L |
Sodium hypophosphite | 15 g/L | 15 g/L | 15 g/L |
Sodium gluconate | 15 g/L | 15 g/L | 15 g/L |
Ammonium sulfate | 15 g/L | 15 g/L | 15 g/L |
Alumina (70 nm) | - | 10-90 g/L | - |
Titania (50 nm) | - | - | 1-6 g/L |
Succinic acid | 0.3 g/L | 0.3 g/L | 0.3 g/L |
Sodium dodecyl sulfate | 0.1 g/L | 0.1 g/L | 0.1 g/L |
Lead acetate | 2 mg/L | 2 mg/L | 2 mg/L |
Time | 60 min | 60 min | 60 min |
Temperature | 90°C | 90°C | 90°C |
pH | 9 | 9 | 9 |
Stirring speed | 150 rpm | 150 rpm | 150 rpm |
- The coating layer was stripped using 10% H2SO4 solution. The specimen was made the anode in an electroplating cell in which the coating layer is dissolved in the solution, which is then diluted to 250 mL with doubly-distilled water.
- The analysis was done using atomic absorption spectrophotometry (Perkin-Elmer 3100, Germany).
- The solution obtained in (1) was further diluted by dissolving 5 mL in doubly-distilled water to 250 mL.
- Nickel standard solutions for the elements to be detected were prepared (1.0 g Ni metal in (1+1) HNO3. Diluted to 1.0 L with 1.0 vol% HNO3) , Ni, air-acetylene flame gases and standard wavelength of 232 nm.
- The phosphorus weight was calculated by subtracting the nickel and alumina or titania weights from the total deposited weight.
dR = dm/dt (1)
The surface morphology of the as-deposited Ni-P-Al2O3 and Ni-P-TiO2 composites on copper was studied using scanning electron microscopy (SEM)(JEOL-5410 attached to an EDX unit). The surface phases and phase changes of the different coated substrates was studied with an x-ray diffractometer (Broker AXS-D8 x-ray diffractometer, ADVANCE, Germany), with a copper target (Cuλ = 1.54060Å) and a nickel filter. The Vickers microhardness of the deposits and the specimen material was measured under a 50-gram load, using a Shimdzu hardness tester. The electrochemical experiments were performed using a Volta Lab 40 (Model PGZ301) with the aid of commercial software (Volta Master 4, V 7.08). A saturated calomel electrode (SCE) and a platinized platinum black were used as the reference and auxiliary electrodes, respectively, with different deposited plated specimens as the working electrode. The electrolyte used in the electrochemical cell was a 3.5% NaCl solution. Volta Master 4 calculates and displays the corrosion rate in µm/yr. This rate is calculated from the corrosion current density icorr, the density D, the atomic mass M and the valence V. The calculation is performed as follows:
• 3270 = 0.01 × [1 year (sec) / 96497.8] and
• 96497.8 = 1 Faraday (Coulombs) (3)
.jpg;maxWidth=600)
The effect of TiO2 content in the bath on the deposition rate of Ni-P-TiO2 alloy is illustrated in Fig. 3. The deposition rate increased with increasing titania content in the bath. Figure 4 shows the TiO2 content in the deposit increased with increasing TiO2 nanoparticle concentration in the plating bath. The nickel films from the alkaline bath contained significantly higher amounts of particles.
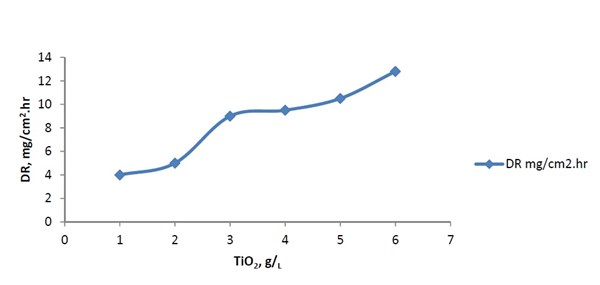
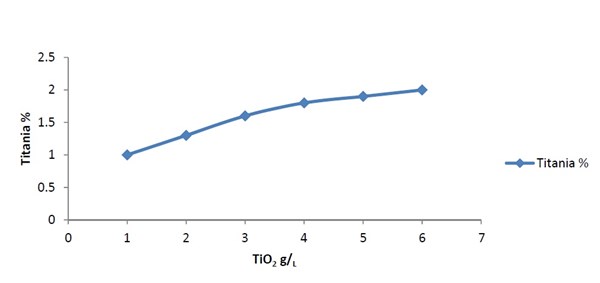
Figure 4 - Effect of TiO2 concentration in the plating bath on the titania content in the Ni-P-TiO2 alloy from a bath containing 25 g/L NiSO4.6H2O, 15 g/L sodium hypophosphite, 15 g/L ammonium sulfate, 0.1 g/L sodium dodecyl sulfate, 0.3 g/L succinic acid, 15 g/L sodium gluconate, time = 60 min, pH 9, temperature = 90°C.
There seems to be a tendency that negatively-charged oxide particles are preferentially codeposited in cathodic processes, at least in solutions containing divalent cations. To explain this counter-intuitive behavior, an electrostatic model has been proposed17-19 that takes into account the charge distribution on the particle and the electrode surface. In the alkaline electrolyte the TiO2 nanoparticles are negatively charged, whereas in the acidic one they bear a positive charge. Under the conditions of the nickel electroplating process, the electrode bears negative excess charges.19 According to the model, negatively charged particles are preferentially attracted by the positive excess charges in the electrolytic part of the electrical double layer of the electrode (EDL). When the particle has come close to the electrode, the shell of adsorbed ions on the particle is stripped off within the EDL of the electrode. Finally the particle becomes incorporated into the growing metal layer.
The major challenges for the codeposition of ceramic particles seem to be the occlusion of a sufficient number of non-agglomerated particles combined with a good dispersion of the particles in the metal matrix. In general, it has been observed that the amount of embedded ceramic particles increases with increasing concentration of suspended particles in the electrolyte.20 Additionally, the reduction of particle size increases the agglomeration tendency of the particles, due to their enhanced surface energy, while decreasing their codeposition content in the metal matrices and the mean grain size of the matrix crystallites.21 Moreover, research has pointed out that the physico-chemical properties of the ceramic particles are crucial to the understanding of the codeposition mechanism of each type of particle.22
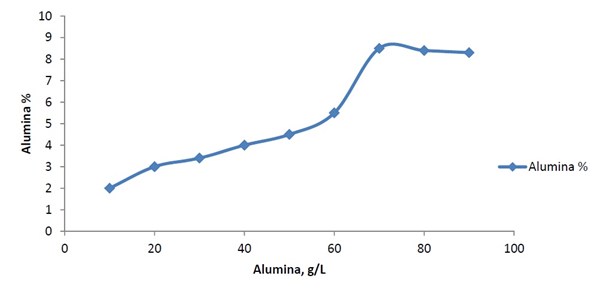
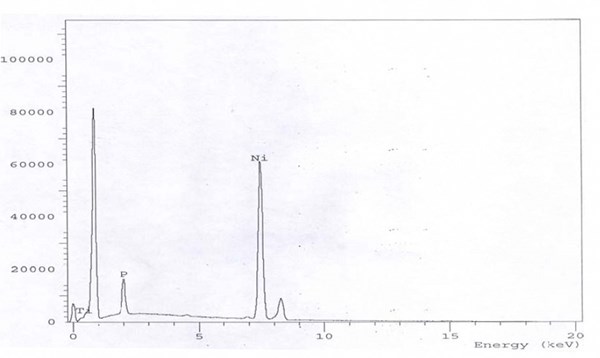
The EDX chart in Fig. 5 shows the pattern 90% Ni-6% P-4% Al2O3 alloy with 40 g/L alumina in the bath. By contrast, a 6.0 g/L TiO2 load in the bath can yield Ni-P-TiO2 with 2% titania as indicated in Fig. 6. The reason that the titania incorporation from the bath (40 g/L ⇒ 4%; 10:1) is higher than alumina (6 g/L ⇒ 2%; 3:1) can be explained from the difference in particle size (50 nm versus 70 nm, respectively). The particle size is a very important factor in the composite coatings formation.8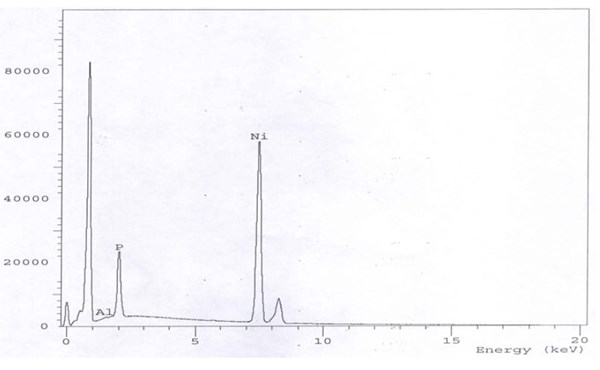
The addition of alumina nanoparticles to the bath obviously affects the crystal orientation of the coatings as indicated from the x-ray diffraction spectra (Fig. 7). The peak width for the alumina (Fig. 7, Sample A) was narrower than the peak obtained for the Ni-P alloy (Fig. 7, Sample C), indicating a change from the amorphous structure. The appearance of the different peaks of Ni-P and Al2O3 without heat treatment is not fully understood. The grain size was 34.5 nm for the alumina sample versus 16.84 nm in the case of the Ni-P alloy. In the XRD pattern of the Ni-P-TiO2 alloy (Fig. 7, Sample B), the reflections corresponding to the (111), (200) and (220) planes of a face centered cubic (fcc) phase of nickel could be observed. The broad peak is evidence of an amorphous structure. The crystal size was 24 nm.
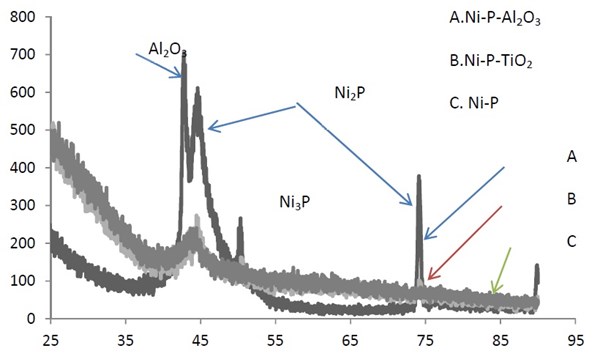
Figure 7 - X-ray diffraction spectra of (a) 90% Ni-6% P-4% Al2O3, (b) 90% Ni-8% P-2% TiO2 and (c) 82% Ni-18% P.


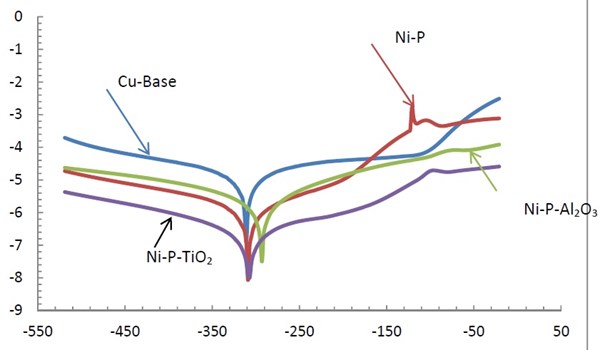
Table 2 - Corrosion data, of the Cu-base, Ni-P, Ni-P-Al2O3 and Ni-P-TiO2 in 3.5% NaCl solutions.
Ecorr (i=0), mV | Icorr, µA/cm2 | Rp kΩ·cm2 | Beta a, mV/decade | Beta c, mV/decade | Corrosion µm/yr | |
Cu-base | -315.0 | 6.2359 | 1.93 | 73.40 | -82.7 | 72.40 |
Ni-P | -225.0 | 0.2848 | 57.64 | 72.74 | -89.7 | 3.296 |
Ni-P-Al2O3 | -233.71 | 0.2584 | 32.323 | 56.517 | -64.0696 | 2.9998 |
Ni-P-TiO2 | -323.9 | 0.17229 | 0.3191 | 57.3 | -71.6 | 2.0170 |
2. A.I. Berner, O.P. Sideleva & V.E. Shub, J. Analytical Atomic Spectrum, 5 (4), 167R (1990).
3. Y.J.M. Park & K.S. Goon, Han’guk Pyomyon Kinghak Hoechi, 24, 222 (1991).
4. O. Berkh, S. Eskin, A. Brener & J. Zahavi, Plating & Surface Finishing, 82 (1), 54 (1995).
5. K.G. Keong, W. Sha & S. Malinov, J. Alloys and Compounds, 334 (1-2), 192 (2002).
6. G.O. Mallory & J.B. Hajdu, Electroless Plating: Fundamentals and Applications, NASF, Washington, DC, 1990; p. 98.
7. G. Jiaqiang, et al., Materials Letters, 59 (2-3), 391 (2005).
8. J.N. Balaraju, C. Anandan & K.S. Rajam, Surf. Coat. Technol., 200 (12-13), 3675 (2006).
9. A.S. Hamdy, et al., J. Appl. Electrochem., 38 (3), 385 (2008).
10. Y. de Hazan, et al., J. Colloid & Interface Sci., 328 (1), 103 (2008).
11. E.A. Abd El Meguid, S.S. Abd El Rehim & E.M. Moustafa, Trans. Inst. Met. Fin., 77, 188 (1999).
12. S.S. Abd El Rehim, S.M. Sayyah & M.M. El Deeb, Appl. Surf. Sci., 165 (4), 249 (2000).
13. S.S. Abd El Rehim, S.M. Sayyah, M.M. El Deeb, Plating & Surface Finishing, 87 (9), 93 (2000).
14. S.M. Rashwan, A.E. Mohamed, S.M. Abdel Wahaab & M.M. Kamel, Mansoura Sci. Bull. A: Chem., 27, 121 (2000).
15. G.O. Mallory & J.B. Hajdu, Electroless Plating: Fundamentals and Applications, NASF, Washington, DC, 1990; p. 386.
16. C. Gabrielli & F. Raulin, J. Appl. Electrochem., 1 (3), 167 (1971).
17. F. Wünsche, A. Bund & W. Plieth, J. Solid State Electrochem., 8 (3), 209 (2004).
18. A. Bund & D. Thiemig, J. Appl. Electrochem., 37 (3), 345 (2007).
19. A. Bund & D. Thiemig, Surf. Coat. Technol. 201 (2007) 7092.
20. I. Garcia, J. Fransaer & J.P. Celis, Surf. Coat. Technol., 148 (2-3), 171 (2001).
21. T. Lampke, et al., App. Surf. Sci., 253 (5), 2399 (2006).
22. G. Vidrich, J-F. Castagnet & H. Ferkel, J. Electrochem. Soc., 152 (5), C294 (2005).
23. Y. Zhou, H. Zhang & B. Qian, Appl. Surf. Sci., 253 (20), 8335 (2007).
Related Content
Online Energy Savings Calculator Promotes Energy Efficiency
An online energy savings calculator from AkzoNobel aims to help customers determine how to reduce energy usage.
Read MoreNASF/AESF Foundation Research Project #123: Electrochemical Manufacturing for Energy Applications – 4th and 5th Quarter Report
The NASF-AESF Foundation Research Board selected a project on electrodeposition toward developing low-cost and scalable manufacturing processes for hydrogen fuel cells and electrolysis cells for clean transportation and distributed power applications. During the reporting period, efforts were focused on planning the overall project work, with the eventual goal of manufacturing an improved design for a Solid oxide fuel cell anode supported flat tube (SOFC).
Read MoreRevolutionizing Battery Production for Electromobility
Fully automated process combines ultrafine cleaning and protective UV coating for battery cells, replacing the need for traditional film wrapping.
Read MoreSpatial Coating Thickness Measurement Solution for Prismatic Battery Cells
Coatmaster AG’s 3D noncontact coating thickness measurement system employs ATO technology to provide spatial measurement of coating thickness.
Read MoreRead Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More












.jpg;maxWidth=300;quality=90)

















