Although the European Union Restriction of Hazardous Substances (RoHS) Directive 2002/95/EC, the Council of September 2000 on End-of-Life-Vehicle (ELV) and the global “move to green” has been viewed by many as a major hassle and the demise of the metal finishing industry, others look at it as necessity and monumental opportunity.
For the chemical suppliers and platers that have embraced this movement, their companies have become stronger and the industry has seen a bit of the “thinning of the herd.” Forward thinking, trend setting companies view “change is inevitable, change is good and change can be very profitable.” Long gone are the days of dumping hazardous, toxic solutions directly into the sewer system. Now, discharge is highly regulated and even the use of many commonly used chemicals is banned or restricted. The two most notable are hexavalent chromium and cadmium. Both of these were mainstays of the metal finishing industry until enactment of the EU’s RoHS-ELV Directives.
As the bar is further raised, chemicals such as cobalt that are commonly used in high performance-thick film chromates are being closely scrutinized due to the hazardous nature of the substance.
Cobalt - Environmental and health concerns
The European Union (EU) has always been a leader and at the forefront when it comes to environmental concerns. The EU was the originator of the Restriction of Hazardous Substances (RoHS) directive which initially restricted six hazardous materials: lead (Pb), mercury (Hg), cadmium (Cd), hexavalent chromium (Cr+6), polybrominated biphenyls (PBB) and polybrominated diphenyl ethers (PBDE).
On September 18, 2000, the European Parliament issued a directive on “End-of-Life-Vehicles” in order to reduce the quantity of waste (including hazardous waste) that occurs when motor vehicles are junked or totaled. Hazardous means substances that are explosive, oxidizing or flammable in the physical environment; or toxic, harmful, corrosive or an irritant to human health.
Article 4(3) and Article 6 of the RoHS directive requires the periodic review of new scientific evidence to consider whether other hazardous substances should be added to the list of prohibited substances. The review was conducted by the Oko-Institute e.V., located in Germany.
High priority substances were selected according to the following criteria:
- Substances of very high concern (SHVC) as defined by REACH (article 57), because they are carcinogenic, mutagenic or toxic for reproduction; persistent, bioaccumulative and toxic or very persistent and very bioaccumulative; of equivalent concern due to endocrine disruptive properties.
- Substances found in humans and biota, raising concerns regarding their long-term harmful effects.
- Substances which can form hazardous substances during the collection and treatment of electrical and electronic equipment, such as during recycling or incineration.
Cobalt and cobalt oxide are listed as “high priority” substances materials being considered for inclusion in the list of RoHS.1
California’s Proposition 65 also known as “The Safe Drinking Water and Toxic Enforcement Act of 1986” is meant to promote clean drinking water and keep toxic substances that cause cancer and birth defects out of consumer products. Proposition 65 has well defined rationale and enforcement as defined as follows:
Rationale and enumerated rights
In addition to amending the California Health and Safety Code, Proposition 65 contained the following language in the 1986 ballot initiative:
"SECTION 1. The people of California find that hazardous chemicals pose a serious potential threat to their health and well-being, that state government agencies have failed to provide them with adequate protection, and that these failures have been serious enough to lead to investigations by federal agencies of the administration of California's toxic protection programs. The people therefore declare their rights:
(a) To protect themselves and the water they drink against chemicals that cause cancer, birth defects, or other reproductive harm.
(b) To be informed about exposures to chemicals that cause cancer, birth defects, or other reproductive harm.
(c) To secure strict enforcement of the laws controlling hazardous chemicals and deter actions that threaten public health and safety.
(d) To shift the cost of hazardous waste cleanups more onto offenders and less onto law-abiding citizens.
The people hereby enact the provisions of this initiative in furtherance of their rights."1 The Legislature's 2003 amendments to Proposition 65 contained the statement that the changes "further the purposes of the Safe Drinking Water and Toxic Enforcement Act of 1986."2
Enforcement
Enforcement is carried out through civil lawsuits against Proposition 65 violators. These lawsuits may be brought by the California Attorney General, any district attorney, or certain city attorneys (those in cities with a population exceeding 750,000). Lawsuits may also be brought by private parties "acting in the public interest," but only after providing notice of the alleged violation to the Attorney General, the appropriate district attorney and city attorney, and the business accused of the violation.
A Proposition 65 Notice of Violation must provide adequate information to allow the recipient to assess the nature of the alleged violation. A notice must comply with the information and procedural requirements specified in regulations. A private party may not pursue an enforcement action directly under Proposition 65 if one of the government officials noted above initiates an action within sixty days of the notice. After 2003, private enforcers must also serve a certificate of merit (statement of expert consultation(s) supporting belief of reasonable and meritorious private action) as a means of preventing frivolous enforcement actions.
A business found to be in violation of Proposition 65 is subject to civil penalties of up to $2,500 per day for each violation. In addition, the business may be ordered by a court of law to stop committing the violation.3 Other penalties may apply, including unfair business practices violations as limited under California Proposition 64 (2004).
The Safe Drinking Water and Toxic Enforcement Act of 1986 requires that the Governor revise and republish at least once per year the list of chemicals known to the State to cause cancer or reproductive toxicity. On April 2, 2010, the list included cobalt metal powder, cobalt oxide and cobalt sulfate.2
So as with hexavalent chromium back in the late 1990s and early 2000s, cobalt, due to being a potential cancer causing suspect agent, is presently on California’s Proposition 65 and listed on RoHS’s “candidate-watch” list for high priority substances.
Demise of hexavalent chromium; rise of cobalt
As ELV put a fairly quick end to the use of hexavalent chromium, an industry mainstay considered to be one of the best finishes for corrosion protection over zinc plate, many suppliers scrambled for a replacement. This replacement would require a general corrosion specification of 120 hr to first white rust (FWR), a clear-clean appearance of blue, yellow, green-rainbow or black and economical to use.
Across the board, every supplier gravitated to cobalt as the backbone replacement component of their new thick film-high performance chromate formulations. The choice of cobalt is threefold: cobalt (1) is readily available, (2) is an easy starting point due to a vast amount of previous research and (3) greatly increases corrosion protection. Basically, existing old style trivalent chromate formulations could be modified to meet the new standards by increasing the chromium content and by adding cobalt. These came at a cost since hexavalent chromates were cheap to manufacture and were used at very low concentrations of 1-2 vol%.
The new high performance-thick film chromates were very expensive to manufacture due to the costly hexavalent to trivalent reduction reaction and the high cost of cobalt salts. These new trivalent chromates were also used at much higher concentrations of 10 vol%.
However, cobalt was the single, most important component that allowed suppliers to meet the ELV Directive and the corrosion protection demands of the automotive industry.
The importance of cobalt is easy to overlook but it did some great things when incorporated into trivalent chromate formulas that other chemical constituents could not do.
Cobalt is a transition metal having similar properties as iron, zinc, chromium, nickel and copper. Whereas, many of the other transition metals cause appearance problems when used in trivalent chromate formulations, cobalt provided performance benefits as well as maintaining the clarity, color and appearance of the chromate film.
A likely candidate would have been iron. However, it significantly lowered corrosion protection and caused yellowing and clouding of the chromate film. Other logical choices were copper, nickel, titanium, vanadium, zirconium and molybdenum. These were expensive, difficult to use during the manufacturing process and in many cases, unstable in the chromate solution.
In the end, cobalt was eventually used in every viable, thick film chromate for passivating a zinc substrate on parts required to meet the ELV Directive.
Problems caused by cobalt
Although cobalt was the life line for suppliers and platers of zinc plated components requiring high end corrosion protection, it also caused many problems. Along with the above mentioned environmental-health hazards, cobalt also caused problems with adhesion of post zinc plate-passivation of powder coat, e-coat, paint and adhesive glues. This coupled with additional expense that cobalt added to the high performance-thick film trivalent chromate process created another dilemma for the zinc plating industry.
Powder coat, paint and e-coat adhesion problems
Powder coating is an excellent surface finish for components requiring superior corrosion protection along with high resistance to chipping. It is ideal for outdoor applications such as patio furniture, garden equipment and barbeques, as well as indoor applications such as computer chassis, kitchen appliances, furniture and cabinets. Powder coating over a zinc plated part with a good chromate makes this one of the most durable of surface finishes.
Prior to environmental shake-ups and the demise of Cr+6 in chromates for zinc plating, adhesion of powder coatings was not typically a problem. Specifications requiring zinc plate, a hexavalent chromate, then followed by powder coating, rarely encountered peeling, flaking, bubbling, out-gassing or other adhesion problems. Hexavalent chromates were excellent base coatings due to their porous nature and their ability to adhere to the fresh electrostatic powder and then strongly bind during the bake-curing process. They also provided additional corrosion protection so the part had a protective three-tier barrier coating (powder coat over the chromate, chromate over the zinc and zinc protecting the steel).
As cobalt containing trivalent chromates transitioned in and hexavalent chromates moved out, the powder coating industry along with paint, e-coat and adhesive glues encountered a myriad of adhesion problems. Powder coaters were hit with a sudden and unexplainable increase in rejections due to coating peel-off, lifting and bubbling. Problems occurred directly out of the bake/cure oven, during shipping, out in the field or at test labs.
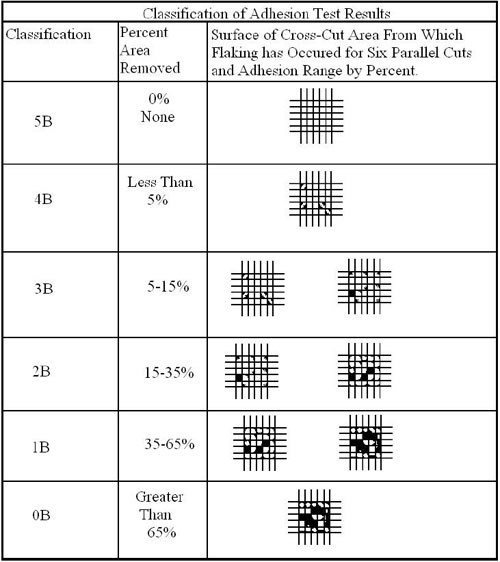
In order to perform, coatings must adhere to the chromate substrate on which they are applied. The standard test procedure used to assess the resistance of the coating to separation is the cross-cut test. This test method, ASTM D3359, uses a procedure where a right angle lattice pattern is cut into the coating penetrating through to the substrate. The cross-cut test is a simple method for evaluating the adhesion of a coated part. The procedure is outlined as follows:
- Make a lattice pattern in the film with the appropriate tool, cutting to the substrate.
- Brush the area with a soft brush or tissue to remove any detached flakes or ribbons of coatings.
- Place the Permacel Tape over the grid and smooth into place by a finger and then with a pencil eraser to ensure good contact.
- Remove the tape rapidly at 180° angle.
- Rate adhesion in accordance with 0B through 5B scale in Table 1.
As failures significantly increased, and parts subjected to ASTM D3359 had results in the 0B to 4B range, the new high performance-thick film chromates were highly suspect. To understand better the adhesion failures and the inability to provide consistent ASTM D3359 Classification 5B results, a closer look of the matrix structure of these cobalt containing trivalent passivates is needed.
The trivalent chromium - cobalt matrix
Standard hexavalent chromates are simple technology containing a hexavalent chromium source, basic commodity salts, organic acids and inorganic acids. These simple Cr+6 formulations made them inexpensive workhorses and provided a porous outer film making them ideal substrates for post-passivate coatings such as powder coat.
The new high performance trivalent chromium film had different characteristics. The film was harder, much less hydrated and contained an additional metal not found in hexavalent chromate films, cobalt.
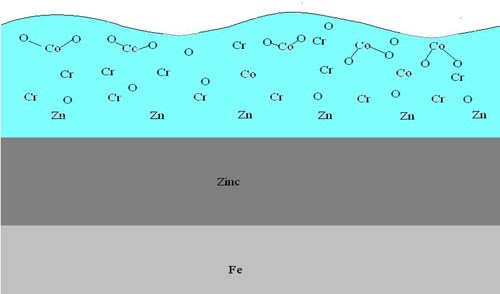
Using glow discharge atomic emissions spectroscopy, a rapid and accurate description of the surface and bulk elemental composition can be determined. The chromate depth profile in Fig. 1 shows cobalt within the trivalent chromate matrix to be most dense at the surface of the chromate film and then significantly dropping off toward the zinc deposit substrate. Also of importance is the high concentration of oxygen at the chromate surface.
As the trivalent chromate layer builds, multiple chemical interactions take place in the solution and at the interface of the zinc layer. These interactions result in metal-oxide formations that deposit on the surface of the zinc, thus building the chromate film matrix. The outer surface of this film contains high levels of oxygen atoms which serve as an ideal base surface for adhesion of topcoats and paints.
If cobalt is present in the trivalent chromate solutions, this oxide-rich surface becomes effectively, “tied-up” by the association of the divalent cobalt atom and the oxygen atoms. The end result is that the oxygen atoms no longer serve as adequate bonding sites for the topcoats. The available oxygen becomes bonded to the cobalt atoms and can no longer serve as active bonding sites for topcoats. The adhesion is poor as the available oxygen bonding sites are taken up by the cobalt. Less available oxygen translates to poor powder coat, e-coat and paint adhesion.
The cross sectional in Fig. 2 provides a better view of the cobalt orientation near the surface of the chromate film.**
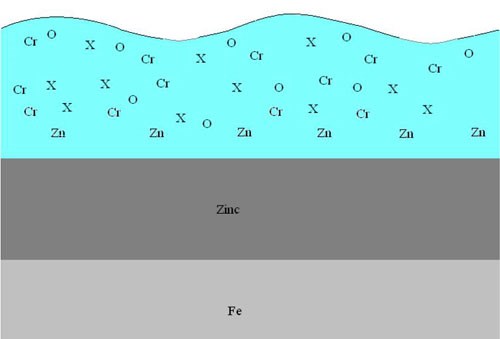
The new cobalt-free technologies use proprietary components (X) that are uniform mixtures within the trivalent chromate matrix. These components allow maximum available oxide bonding sites for post-passivate coatings. The proprietary components also have no or very little affinity to bond to oxygen, thus leaving an ideal substrate for coatings. Figure 3 shows the X components orientation within the chromate matrix.***
Cost and performance are a vital part of any new trivalent chromate. The metal finishing industry, specifically anyone using trivalent chromates containing cobalt, took a hit during 2008. After reaching the peak in June 2008, the price of cobalt salts increased by over 120% from the normal average. Metal finishing suppliers and applicators were locked-in on price and had to bite the bullet, taking significant losses during the cobalt price upswing. Typically, it was very difficult for suppliers to increase the price of their trivalent chromates and for platers to raise price quotes on work.
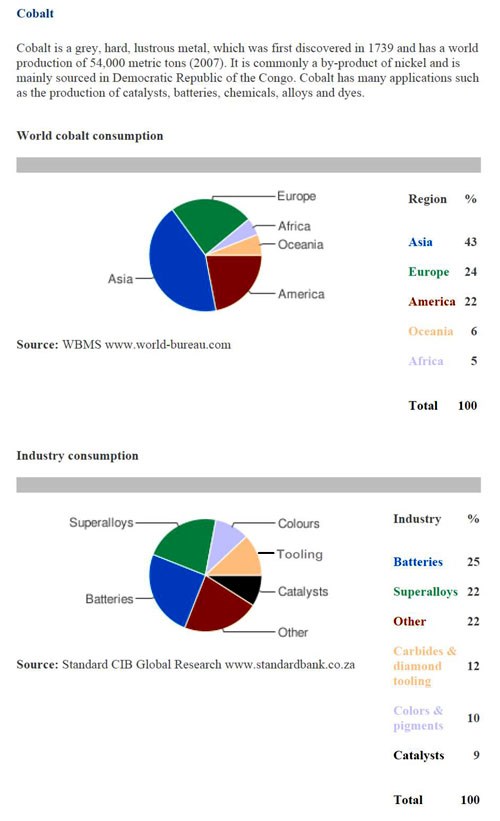
Although cobalt salt prices have stabilized for now, all indications are cobalt and its salts will eventually rise. Demand is up with China being one of the largest users. Since being added to the London Metal Exchange (LME) in February 2010, pricing and use are much easier to track. Figure 4 shows the consumers and industry use for cobalt.
Metal finishers received “no breaks” from the automotive industry when the ELV directive mandated, “no more hexavalent chromium,” over zinc plated components. Initially, suppliers were asked to reduce the cost of their chromates by 10-15% and increase the general salt spray requirements of 24-96 hr to first white rust (FWR) to a rigid standard of 120 hr to FWR. Price concessions were later given as suppliers scrambled to develop trivalent chromates capable of exceeding the old hexavalent chromate specifications. However, the performance requirements remained 120 hr to FWR as this became the automotive industry standard.
This is a much more competitive world compared to the RoHS-ELV directives of the early 2000s. New and innovative technologies will have greater emphasis on lowering production costs yet increasing corrosion protection.
In order to achieve both cost-performance goals, suppliers and formulators must take a hard look at their present product mix. The two largest expenses in today’s trivalent chromates are the trivalent chromium source and cobalt salts. Typically, these two items account for roughly 80%+ of the total product cost.
So, the logical place to start is to reduce the trivalent chromium content and develop some interesting technology to replace cobalt. This has to be done without sacrificing performance and appearance. Actually, any new innovations for this industry should also have performance benefits, so increased corrosion protection is important.
This most likely will not happen by using simple commodity chemicals. Cobalt-free technologies of today’s world require a lot more innovation than simply dropping in one metal salt. Cobalt replacement will require multiple component formulations, whereas one component provides corrosion protection, a second component provides clarity/color to the chromate film and the other components offer self-healing characteristics, and the ability to harden when baked.