Combining Ecoat and Plating for Next Generation Edge Protection
A method by which a high-purity and well-coalesced layer of aluminum metal is electroplated onto a steel substrate from an ionic liquid electrolyte.
Efficient corrosion protection of the sharp edges of manufactured steel fasteners has long been a goal for coaters and coating formulators. Numerous commercial processes exist to achieve this goal, but all involve large tradeoffs between cost/practicality and performance. A method is described and proven by which a high-purity and well-coalesced layer of aluminum metal is electroplated onto a steel substrate from an ionic liquid electrolyte. The aluminum plated fastener is then coated with a layer of cationic epoxy electrocoat. This combination provides exceptional efficiency, adhesion and corrosion protection – especially on sharp edges – while the process similarities between the aluminum plating and e-coat make the concept an ideal candidate for commercialization.
The automotive and industrial benchmark for cost-efficient corrosion protection of steel is a layer of zinc phosphate followed by a layer of cationic epoxy electrocoat. The electrocoat can serve either as a stand-alone coating or as a primer for successive paint layers. Both the phosphating and electrocoating are high-throughput, high-efficiency processes which allow in-line coating application with minimal labor input per square meter finished, and very little waste.
This process provides excellent corrosion resistance, particularly over smooth, flat or gently curved surfaces. The sharp edges prominently featured on steel fasteners, however, are a different story. Thermosetting by nature, all electrocoat products must go through a semi-liquid ‘melt’ stage (McMillan, 2002) before building enough heat to chemically unblock their crosslinkers, evaporate the blocking agent, and finally crosslink, or ‘cure.’ This melt phase is necessary for the coating to achieve optimum coalescence before cure. Without it, the cured film would be rough, low gloss, and porous, ruining both its aesthetic and protective properties. But too much flow before cure results in “pull-away” from sharp edges, leaving little or no protective layer after crosslinking (Sample, 2007).
One way to battle loss of sharp edge protection during cure is to reduce or eliminate the edges themselves before coating. This approach can be very effective, but also very expensive in terms of engineering and handling. The second way is through electrocoat formulation – designing the coating to achieve the desired balance of appearance and edge protection (Corrigan, 1992). This approach can also be effective, but it poses technological challenges and adds formulation cost. The large-batch, scale efficiencies used by electrocoat manufacturers also limit tailoring products to the needs of the individual industrial coater.
This article describes a third approach – electroplating a layer of pure aluminum metal between the base steel substrate and the electrocoat layer.
Deposition of Aluminum from Ionic Liquid
Aluminum Plating Background
Unlike more easily electroplated metals (tin, silver, zinc, etc.), the reduction potential of aluminum in aqueous solution (Al3+) is more negative than the reduction potential for water. This means that any attempt to electroplate aluminum from an aqueous medium will be unsuccessful, as water will simply electrolyse to pure hydrogen (at the cathode) and oxygen (at the anode) gases while the aluminum salt remains in solution.
If aluminum clad steel is desired, an alternate means of aluminum deposition must be sought. A technological process was developed over the latter half of the 20th century by Siemens and the Max Planck Institute, and then commercialized in 1995 by a new American company called Alumiplate. The process involves electrodeposition of aluminum from an organic bath comprising an aluminum salt, a stabilized electrolyte, and organic solvent.
In the Alumiplate process, the obvious technical advancement of electroplating aluminum onto steel is tempered somewhat by the need for flammable, volatile organic solvent in the plating bath. Using an ionic liquid electrolyte, however, eliminates the need for volatile organics in the deposition bath, reducing both the fire hazard and the VOC-management demand on the inert-atmosphere plating system. Zero VOC in the plating bath means less evaporation, lower emissions, and no need for replacement or monitoring over time.
Ionic Liquids
Ionic liquids are a broad class of chemical compounds which are both ionic in nature and liquid in neat form at T < 100° C (SERDP, 2011). Importantly, most ionic liquids also exhibit nearly zero vapor pressure. This property is very interesting from an industrial perspective because it allows for the conservation of raw materials, since none can be lost to evaporation.
For comparison, common ionic solids often involve either a metal or small organic cation coupled with an anion, comprising either a simple halogen or transition element in any of several oxidation states. Sodium Chloride (NaCl) is one of the most familiar examples. Copper nitrate (Cu(NO3)2) and Ammonium Sulfate ((NH4)2SO4) are two more.
The atomic size and electronic structure of this kind of compound typically results in a pure material which forms a highly-ordered, crystalline solid. They usually exhibit good solubility in strongly polar solvents like water, and limited solubility in non-polar, organic solvents. In contrast, ionic liquids generally involve larger organic cations, and larger, more complex anions. The classic example is the imidazolium cation, shown in figure 1 compared to neutral imidazole.
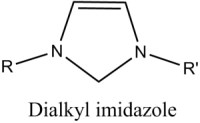
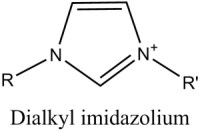
Prior art has shown that alkylimidazolium halides provide a good balance of low temperature viscosity, conductivity, vapor pressure and solvating properties, and therefore are an excellent ionic electrolyte for the electroplating of aluminum (Wilkes, 1982). Several variations of commercial plating liquids are currently available in the US (Sigma-Aldrich, 2014).
Aluminum Plating
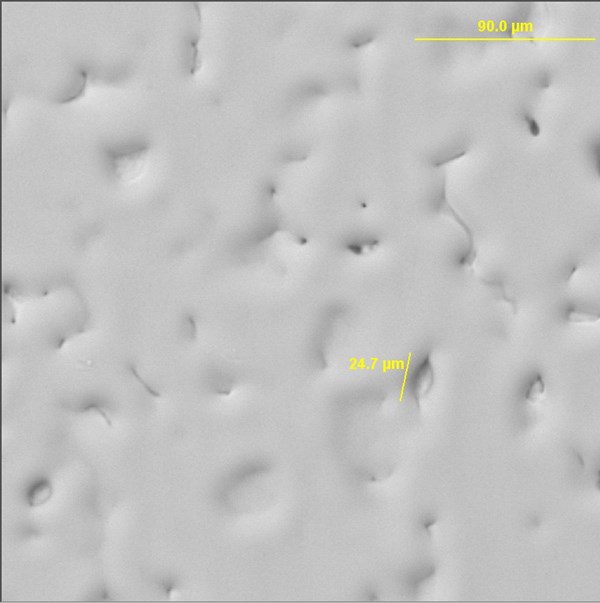
Despite initial reports of wide operational plating windows, substantial experimentation was needed to achieve adherent and consistent layers of electrodeposited aluminum. Bath temperature, maximum voltage, voltage profile, current density, anode quality, and substrate cleanliness all play critical roles in the deposition of a coherent layer of plated aluminum onto steel. The wrong combination of parameters will lead to staining, ionic liquid degradation, loss of electrical efficiency, poor adhesion and other defects (figure 2).

Once deposition parameters are optimized, however, high-quality layers of aluminum can be successfully and repeatedly deposited onto bare steel test panels (figure 3).

Electrocoat Application
Established ionic liquid/aluminum plating literature (BASF, 2013) focuses mostly on the aluminum itself as the key protective layer for the underlying steel substrate. Effort has been put forth to produce the optimum combination of thickness, consistency and aesthetics from a single plated layer of aluminum. Using plated aluminum as the sole corrosion protective layer on a steel fastener poses two key problems. First, while the aluminum is more resistant than steel to neutral-electrolyte accelerated corrosive attack, it is susceptible to high or low pH chemical attack. Second, most fasteners are required to meet a specific torque/tension relationship, where a fixed tensile clamping strength is consistently developed after driving the fastener to a known and controlled torque. Friction-modified coatings are often used to optimize this property, but plated aluminum typically offers no coefficient-of-friction benefit. Both of these problems are easily addressed by a layer of cationic epoxy electrocoat, formulated specifically for use in the steel fastener market.
Designing a plated aluminum layer is simplified greatly when a layer of electrocoat is the planned next step. Much aluminum cladding literature revolves around finding the right operational parameters to optimize the appearance of the aluminum coating to produce a layer that has the smooth, reflective look of solid aluminum or aluminum alloy. An SEM image of a plated aluminum layer meeting these characteristics is shown in figure 4.
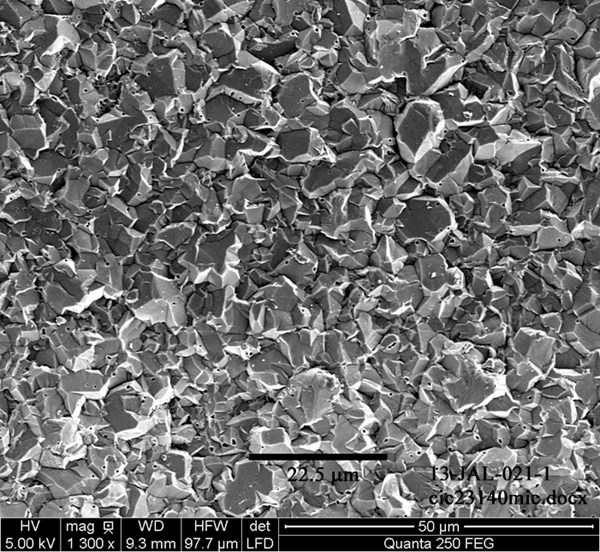
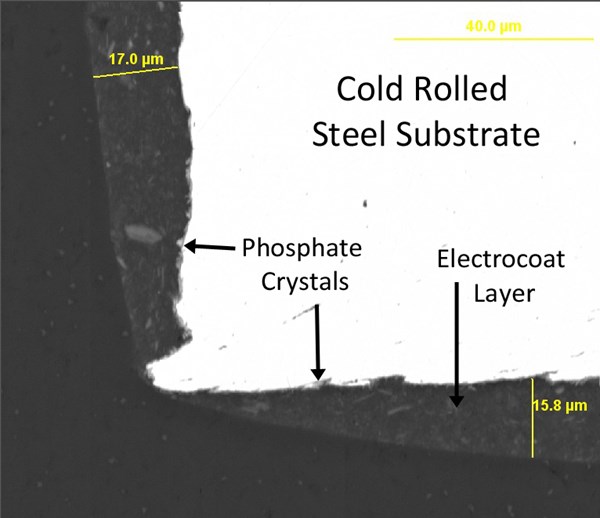
When an organic coating layer is to be applied, however, the very properties that give a pleasing aluminum appearance (smoothness, lack of profile, lack of porosity) are counterproductive for adhesion of the organic film. Ideal substrate condition for paint adhesion is best exemplified by the classic zinc phosphate coating. A high quality zinc phosphate layer provides excellent adhesion to the underlying steel, and its complex, crystalline surface dramatically increases the total surface area on a micron-scale (Rausch, 1990). The ideal phosphate crystals are tightly packed and generally 3-10 mm in individual crystal size (figure 5). They may be either nodular or needlelike in shape, depending on the phosphate makeup and application method.


Achieving a perfectly smooth, bright and coherent layer of plated aluminum on steel poses a challenge, but generating a plated aluminum layer that shares physical characteristics with zinc phosphate is more straightforward (figure 6). The depicted morphologies lead to excellent adhesion and corrosion resistance after the aluminum is electrocoated with an organic film.

Corrosion Testing
As previously discussed, traditional electrocoat products have a tendency to pull away from sharp edges during cure, significantly reducing their corrosion resistance at those spots. Even over zinc phosphated edges, red rust can appear surprisingly quickly in accelerated testing. Figure 7 shows the cross section of a phosphated and electrocoated steel edge under SEM, and figure 8 shows the corrosion results on this edge after only 48 hours in ASTM B117 salt fog testing (unscribed).

When the zinc phosphate layer is replaced by a layer of aluminum plated from ionic liquid, excellent results are achieved in both SEM cross section (figure 9) and accelerated corrosion testing (figure 10). With this process, sheared-edge steel panels lasting as long as 2500 hours in B117 corrosion testing with minimal signs of corrosion can be obtained.
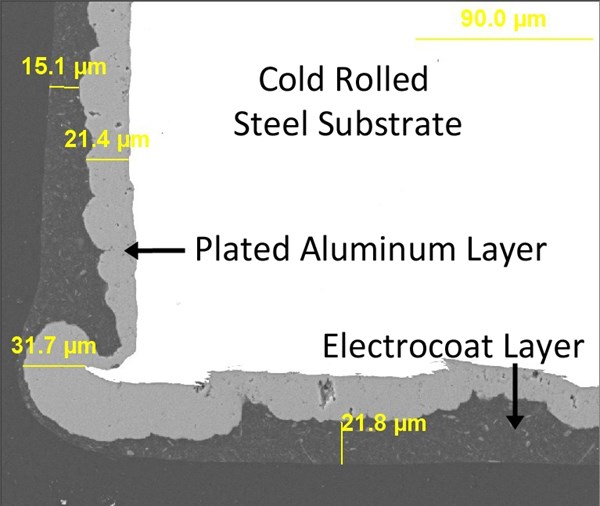

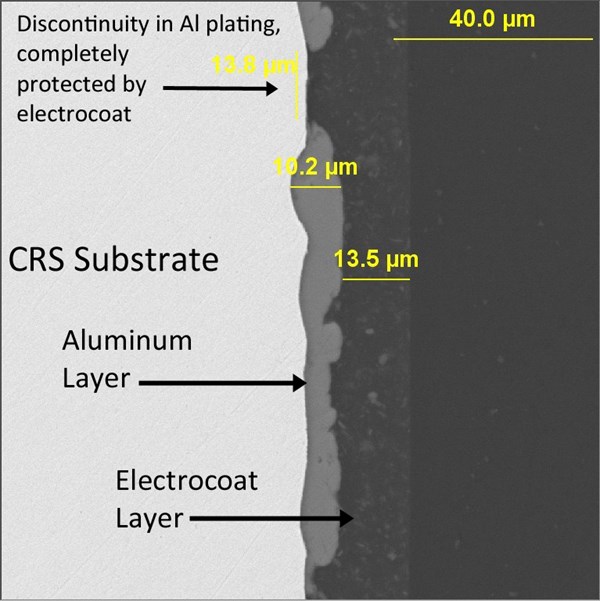
Figure 11 demonstrates in cross-section how well a plated aluminum layer and electrocoat complement each other. The aluminum builds over sharp steel edges with excellent efficiency, while any minor discontinuities in the plating are easily sealed over and protected by the electrocoat. Any necessary slip modification can be formulated into the electrocoat, providing the torque/tension characteristics demanded by the modern fastener coating industry.
Conclusions
Opportunity exists for improved corrosion control in today’s aerospace, automotive and industrial fastener markets. The sharp edges inherent in fastener design, and the processing limitations of hardened steel due to hydrogen embrittlement concerns pose challenges that are addressed by combining aluminum plated from ionic liquid with a sealing-layer of cationic epoxy electrocoat. The automated, bulk application style (Inoue, Ohnuma, Inomata, & Miyadera, 2012) of both processes provide synergies that will help increase efficiency and control costs. The ideal system will be designed to feed batches of fasteners from cleaning and preparation, through aluminum plating and finally into electrodeposition and cure with no need for physical handling of the fasteners between processes. This concept can also be easily adapted for use with any electrocoat line where dramatic improvement in corrosion protection of sharp, steel edges is desired.
Ongoing research is focused on plating optimization, cost minimization, and real-world demonstration of the concept. This paper is based upon work supported by the US Army under contract W56-HZV-09-C-0601. Any opinions, findings and conclusions or recommendations expressed in this paper are those of the author and do not necessarily reflect the views of the Army.
Jon Love is employed by PPG Industries, Inc. He has experience in coatings manufacturing (Quality) and has spent most of his 20 year career formulating electrodeposition coatings. His current responsibilities include supporting numerous US Government and academic efforts to control corrosion over a wide range of metallic substrates. Questions can be directed via email – jlove@ppg.com.
Bibliography
BASF. (2013, February 7). Ionic Liquids as Electrolytes. Retrieved February 7, 2013, from www.basionics.com: http://www.basionics.com
Corrigan, Z. (1992). Patent No. 5,096,556. USA.
Inoue, M., Ohnuma, T., Inomata, T., & Miyadera, T. (2012). Patent No. US2012/0205249A1. US.
McMillan, R. (2002). Determination of Electrocoat Melt Viscosity and Cure Characteristics. Electrocoat 2002 (p. 151). Orlando, FL: The Electrocoat Association.
Rausch, W. (1990). The Phosphating of Metals (English translation). Teddington, Middlesex: Finishing Publications, LTD.
Sample, C. (2007). High Edge Coverage Electrocoat Development for Enhanced Corrosion Protection. Tri-Services Corrosion Conference. Denver, CO: NACE.
SERDP. (2011, October 27). WPSON-13-02. Ionic Liquids Technology Statement of Need. Arlington, VA, USA: SERDP/ESTCP.
Sigma-Aldrich. (2014, April 16). Ionic Liquids. Retrieved April 16, 204, from www.sigmaaldrich.com: http://www.sigmaaldrich.com/chemistry/chemistry-products.html?TablePage=16255866
Wilkes, L. W. (1982). Dialkylimidazolium Chloroaluminate Melts: a New Class of Room-Temperature Ionic Liquids for Electrochemistry, Spectroscopy and Synthesis. Inorganic Chemistry. USA: American Chemical Society.
Related Content
An Altruistic Growth Strategy Puts People First
Professional Plating emphasizes investing in its team and fostering a supportive environment on the shop floor.
Read More10 Ecoat Best Practices
Following this list of guidelines can help to increase the performance, cost effectiveness and quality for your ecoat operation.
Read MoreSustainability, Electrification and Mobility
Industry events like ECOAT are good indicators of the trends that are top of mind for those in manufacturing.
Read MoreTake Full Advantage of Industry Events
As travel plans ramp up for the year, what industry events will you attend? Products Finishing offers a quick look at some of the upcoming opportunities for 2024.
Read MoreRead Next
The Profit Hunt: Finding the Hidden Cost of Electrocoat
Determining real costs means competing in deflationary times.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More












.jpg;maxWidth=300;quality=90)










