
Figure 1: Crazing in a hard anodized layer, LOM 25X magnification Photo Credit: All images courtesy of AluConsult
Q: What is the cause of crazing and cracking when anodizing aluminum parts?
Talking about crazing and/or cracking of the aluminum oxide formed by anodizing of aluminum only makes sense after distinguishing if the two words are the same or not.
To do this, we have to look into the various situations that can cause crazing/cracking of anodic coatings.
Crazing or Cracking
According to Wikipedia1, “Crazing is the phenomenon that produces a network of fine cracks on the surface of a material, for example in a glaze layer.”
Crazing of aluminum oxide shows under magnification a very fine spiderweb (Fig. 1), but can often also be seen by the naked eye.
The aluminum oxide has a high heat resistance, but still, fine cracks can be observed when the anodized aluminum part is exposed to temperatures around 80-100°C (176-212°F).
The major cause for this crazing is the difference in the coefficient of thermal expansion between the oxide coating and the aluminum substrate.
The thermal expansion coefficient of the aluminum metal is relatively large compared to other metals. Pure aluminum (99.996%) will, between 20-100°C (68-212°F), have a value of 23 10-6/°C (13.1 x 10-6/°F)2.
This should be compared to the thermal expansion coefficient of the anodic coating which is almost 5 times less with a value of 5 x 10-6/°C.
Crazing of the oxide layer is caused by thermal stresses but can also be introduced by mechanical stresses in production processes such as bending.
Cracking of the oxide layer shows up as cracks through the oxide layer down to the base metal. So the main difference between crazing and cracking is if the spider web is only on top of the coating whereas on the other hand cracking will break through to the base material, (Fig. 2).
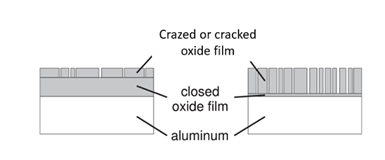
Figure 2: Drawing of crazing and cracking
Crazing will normally be in the top coating whereas cracks due to microstructural and mechanical stress can go more or less through the coating and down to the aluminum metal.
Because of a more compact nature of the aluminum oxide formed during the anodizing process, compressive stresses are introduced into the coating during the process. Leaving the aluminum metal below with a lot of tensile stresses introduced can start fatigue cracks and lead to stress corrosion cracking (SCC). This susceptibility is more pronounced for alloys in the 7000 series containing zinc.
Cracking is often seen when the radius of corners is lower than 90 degrees, forming a very sharp angle. This is especially important when hard anodizing, creating thicker and more dense layers.
The anodic oxide layer will always have some cracks, so it is important to minimize these and make sure that they are not penetrating down to the bare aluminum metal.
Influencing from parameters
The tendency to crazing increases as the hardness of the anodic coating increases, that is a known fact, but is it because of thickness, sealing method or anodizing parameters?
The crazing defects are usually found on anodized aluminum which has been exposed to high temperatures or any considerable sudden changes in temperature. The temperature to which cracking becomes visible is dependent on the thickness. Thick films are more prone than thin films to crack.
The type and degree of sealing has an influence on this crazing phenomenon, reducing the temperature at which thermal crazing occurs. During the hot water sealing process, the compressive stress becomes tensile. Proceeding the sealing greatly reduces the tensile stress and after the sealing, the compressive stress is back again. This stress will decrease the temperature where cracks happen.
During the anodizing process, higher current density increases the possibility of the crazing defect and higher temperature in the anodizing tank will decrease the probability of crazing. Colored parts — especially the electrolytic colored parts — tend to crack less than clear anodizing.
If you find this topic interesting and have seen it on your hard anodized parts, then attend the International Hard Anodizing Symposium in September (www.ihanodizing.com/events) — I will have a presentation and discussion about this annoying defect.
References
- https://en.wikipedia.org/wiki/Crazing
- Davis, J.R. Metals Handbook Desk Edition. ASM, 1998.
About the Author

Deacon
Photo Credit: AluConsult
Anne Deacon Juhl
Anne is the owner of AluConsult and
Anodizing School.
Visit aluconsult.com
Related Content
Trivalent Chrome Overview
As the finishing industry begins to move away from the use of hexavalent chromium to trivalent chromium, what factors should finishers consider as they make new investments? Mark Schario, chief technology officer for Columbia Chemical offers a helpful overview of this complicated topic.
Read MoreFinishing Systems Provider Celebrates 150 Years, Looks to Future
From humble beginnings as an Indiana-based tin shop, Koch Finishing Systems has evolved into one of the most trusted finishing equipment providers in the industry.
Read MoreA Smooth Transition from One Anodizing Process to Another
Knowing when to switch from chromic acid anodizing to thin film sulfuric acid anodizing is important. Learn about why the change should be considered and the challenges in doing so.
Read More10 Anodizing Best Practices
Following this list of guidelines can help to increase the performance, cost effectiveness and quality for your anodizing operation.
Read MoreRead Next
Understanding the Hidden Costs and Benefits of Anodizing
Anodizing has capital costs and benefits beyond the machines — companies evaluating in-house systems should examine them carefully before committing.
Read MoreAvoiding White Spots Caused by Galvanic Corrosion on EN AW 2024
Four approaches to troubleshoot white spots that appear because of galvanic corrosion.
Read MoreIs a Cold Seal Right for Your Anodizing Operation?
Exploring the advantages, challenges and best practices of cold seal processes.
Read More












.jpg;maxWidth=300;quality=90)









