Cyanide Destruction: A New Look at an Age-Old Problem
Cyanide in mining and industrial wastewaters has been around from the beginning, including electroplating processes. This presentation reviews a number of current processes, and in particular, offers new technologies for improvement in cyanide destruction by the most common process, using sodium hypochlorite.
Editor’s Note: The following is a synopsis of a presentation given at NASF SUR/FIN 2018, in Cleveland, Ohio on June 4, 2018 in Session 3, Waste Management: Perspectives Connecting Finishers. A pdf of this brief can be accessed and printed HERE; the complete Powerpoint presentation is available by clicking HERE.
ABSTRACT: Cyanide in mining and industrial wastewaters has been around from the beginning, including electroplating processes. This presentation reviews a number of current processes, and in particular, offers new technologies for improvement in cyanide destruction by the most common process, using sodium hypochlorite.
Introduction
Cyanide chemistries in mining, as well as industrial process wastewaters, including plating operations, have been used since before the start of the industrial revolution. Plating baths derived from cyanide chemistries have been an efficient and economical means of depositing certain metals in commerce. However, issues of toxicity and occupational health have rendered them undesirable, and the search for substitutes has proceeded apace in recent years. However, there remain applications for which cyanide chemistries provide the only means of meeting the property and performance specifications required. Consequently, the effective destruction of cyanide wastewaters is still an important issue to the surface finishing industry
Review of cyanide destruction processes
There are a number of cyanide destruction process in use today. These include:
- Alkaline chlorination
- Biological treatment
- Caro’s acid
- Hydrogen peroxide
- Sulfur dioxide and air
Alkaline chlorination
The most common form of cyanide destruction for plating processes involves alkaline chlorination. Traditional treatment involves a high pH of 10.5, and a high oxidation-reduction potential (ORP) of +600 mV. It is a chemically heavy process, using approximately 23 gallons of 12.5% sodium hypochlorite solution to destroy one ounce of cyanide. Sodium hypochlorite usage at this rate is quite high.
The first step in the process produces cyanogen chloride:
(1)
The cyanogen chloride then hydrolyzes to form cyanate:
(2)
The second step involves the hydrolysis of cyanate ions to form ammonia and carbonates:
(3)
Our new proprietary technology addresses a number of problems associated with pH and hypochlorite usage.*** The operating pH is considered reduced, at 9.5. Most importantly, the advanced technology reduces the equivalent amount of sodium hypochlorite required by 94%, at 1/16 the level of the traditional process.
Biological treatment
Biologic treatment through the use of bacteria has been used as an effective means of cyanide destruction. Aerobic bacteria, including pseudomonas, alcaligenes, achromobacteria and others, are used to form cyanates through oxidation:
(4)
The cyanate ion is biologically converted to ammonia and bicarbonate:
(5)
Caro’s acid
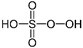
Skeletal formula of peroxymonosulfuric acid
Caro’s acid, or peroxymonosulfuric acid (H2SO5), was introduced in the early 1990s as a cyanide destruction agent. It is produced by an exothermic reaction involving hydrogen peroxide and sulfuric acid. It is very unstable and needs to be used immediately, as the cyanide destruction is a rapid one, reaching completion in just a few minutes:
(6)
The oxidation of cyanide to cyanate involves a one-to-one molar ratio in this process. The highly reactive chemistry is a major disadvantage in using this technology on the scale of plating operations.
Hydrogen peroxide
The hydrogen peroxide process is widely used at steel hardening facilities, primarily as a case-hardening process for low carbon steels. Typically, the process involves a molten salt bath of sodium cyanide operating in the range of 1600-1750°F. Again, environmental issues have led to considerably less usage of this technology.
Cyanide destruction by hydrogen peroxide requires a catalyst such as copper to increase the reaction rate. The metals bound by the cyanide must be precipitated as a metal hydroxide. Once again cyanide is converted to cyanate:
(7)
For the bound metals,
(Solid) (8)
Sulfur dioxide and air
There are two patented versions of this process, held by Inco and Noranda, related to nickel processing. In particular, the mining operations use the process to treat tailing slurries. The process is effective for treatment of free and weak-acid dissociable (WAD) cyanides, and a catalyst is required to achieve a practical reaction rate. The reactant from air is oxygen. Again, cyanide is converted to cyanate:
(9)
For the bound metals,
(10)
The optimal process pH is in the range of 8-9. The sulfur dioxide can be in the form of liquid sodium dioxide or derived from sodium sulfate or sodium metabisulfite.
Summary
The use of cyanide chemistry in industrial processes, while posing environmental and health problems, is still in use in many mining and industrial arenas, including plating operations, owing to the fact that specific alternatives are few or non-existent. This paper reviews technologies used in the mining and surface finishing industries.
Cyanide destruction is the primarily cyanide destruction technology in electroplating, and generally involves the use of sodium hypochlorite chemistry. A process is discussed here which allows operation with reduced alkalinity and a significant reduction in sodium hypochlorite usage, reducing a number of safety and chemical consumption concerns.
About the author

Robin Deal is a Wastewater Field Service Technician with Hubbard-Hall, Inc., in Inman, South Carolina. She holds a Physical/Chemical Waste Water Operator’s license for North Carolina and is currently working toward a Bachelor of Science degree from the North Carolina Agricultural and Technical State University (2017-2020).
*Compiled by Dr. James H. Lindsay, Technical Editor - NASF
** Corresponding author
Robin Deal
Technical Field Service
Hubbard-Hall
1101 Compton Bridge Road
Inman, SC 29349
Phone: (864)-472-2258
E-mail: rdeal@hubbardhall.com
***AquapureTM Wastewater Treatment Processes, Hubbard-Hall, Inc., 1101 Compton Bridge Road, Inman, SC 29349.
Related Content
NASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 9th Quarterly Report
The NASF-AESF Foundation Research Board selected a project addressing the problem of PFAS and related chemicals in plating wastewater streams. This report covers the ninth quarter of work (January-March 2023). In this report, we describe our work on evaluating the performance of PFAS degradation by electrooxidation using surface fluorinated Ti4O7 anodes in batch mode.
Read MoreNASF/AESF Foundation Research Project #120: Electrochemical Destruction of Perfluorooctanesulfonate in Electroplating Wastewaters - April 2022-March 2023
This NASF-AESF Foundation research project report covers project work from April 2022 to March 2023 at the University of Illinois at Chicago. The overall objective of this work is to utilize a cost-effective reactive electrochemical membrane (REM) for the removal of PFAS from synthetic electroplating wastewater. Initial results for the oxidation of PFOA with three different catalysts are discussed.
Read MoreExplore Cleaning Chemistry, Metal Finishing Applications and Wastewater Treatment Solutions
Hubbard-Hall Celebrating 175 years of excellence, Hubbard-Hall presents chemistry and equipment.
Read MoreNASF/AESF Foundation Research Project #121: Development of a Sustainability Metrics System and a Technical Solution Method for Sustainable Metal Finishing - 15th Quarterly Report
This NASF-AESF Foundation research project report covers the twelfth quarter of project work (October-December 2023) at Wayne State University in Detroit. In this period, our main effort focused on the development of a set of Digital Twins (DTs) using the Physics-Informed Neural Network (PINN) technology with application on parts rinsing simulation.
Read MoreRead Next
Episode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More











.jpg;maxWidth=300;quality=90)









