Decorative Trivalent Chromium Deposits Applied by Barrel Electroplating
With the wide acceptance of the benefits of decorative trivalent chromium plating, there is a growing demand to successfully adapt this technology to barrel plating. Traditionally, trivalent chromium deposits were not commercially viable using barrel electroplating. Factors such as barrel design, cathode contact, anode placement and current density must be considered when providing a trivalent chromium barrel electrodeposit. This presentation outlines trivalent deposits applied in a barrel and examines variables in operating and electrolyte compositions.
Editor’s Note: The following is a paper based on a presentation given at NASF SUR/FIN 2019, in Rosemont, Illinois on June 3, 2019 in Session 1, Automotive 1: Innovative Decorative Finishes. A pdf of this brief can be accessed and printed HERE; the complete Powerpoint presentation is available by clicking HERE.
ABSTRACT: With the wide acceptance of the benefits of decorative trivalent chromium plating, there is a growing demand to successfully adapt this technology to barrel plating. Traditionally, trivalent chromium deposits were not commercially viable using barrel electroplating. Factors such as barrel design, cathode contact, anode placement and current density must be considered when providing a trivalent chromium barrel electrodeposit. This presentation outlines trivalent deposits applied in a barrel and examines variables in operating and electrolyte compositions.
Introduction and history
In 1939, the U.S. Bureau of Mines began research and developed a electrowinning process in the 1940s. Union Carbide also began research on trivalent chromium at this time. Together they broke down many obstacles in the art of trivalent chromium plating.
In the early 1970s, Albright Wilson further developed the process. Their contributions were additives to prevent the formation of hexavalent chromium and a method to precipitate metallic impurities. The Albright Wilson chemistry was a mixed sulfate/chloride electrolyte. There were other developments that were sulfate only electrolytes.
Trivalent chromium plating has proven to be a viable alternative to hexavalent chromium in both cost and performance in decorative rack plating for over 20 years.
Trivalent chromium barrel plating - challenges and approach
Until recently, chromium was never successfully applied in a decorative barrel process simply due to the limitations of hexavalent chromium electrolytes. Hexavalent chromium is prone to burning in high current density areas, and hexavalent chromium does not tolerate current interruption. For these reasons alone, it is virtually impossible to plate hexavalent chromium in a barrel.
Barrel plating of chromium is highly desirable. Small parts do not lend themselves to manual racking and throughput is slow in decorative rack processes. Plating small parts such as fasteners, screws, sockets in a barrel could yield drastic savings in labor/ throughput. With the problems inherent in hexavalent chemistry, the possibility of using trivalent chromium chemistry for barrel plating was pursued.
However, despite the advantages of increased current efficiency over hexavalent processes - plus all of the environmental advantages - challenges remain. The current efficiency of trivalent chromium is still quite low compared to other plating processes (copper, nickel, zinc, ...) leading to chromium coverage that is less than that of nickel. Further, the current density and voltage needed is more than nickel.
Despite these shortcomings, the first step was to try the current trivalent chromium rack processes solution in a barrel. The performance could be evaluated and, based on the results, the process could be modified to meet barrel plating needs.
Results with rack formulation
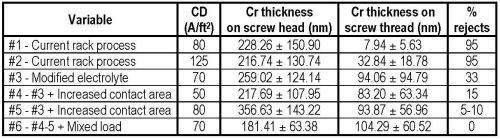
As a starting point, a load of standard screws was barrel plated using the rack process formulation at a current density of 80 A/ft2. Plating time was 5 min. Under those conditions, greater than 95% of the screws were rejects. The plating defects showed incomplete nickel coverage and some darkening of the underlying nickel deposit. The deposition rate was approximately 40% slower than rack plating at 45 μm/min. As a reference the trivalent rack process typically deposited at 100 μm/min. The plate distribution was very unsatisfactory with an average thickness (± one standard deviation) of 228.26 ± 150.90 nm on the screw head and a very low value of 7.94 ± 5.63 nm on the threads. Clearly, the existing operating parameters used for rack plating were not acceptable for barrel plating.
Our second test involved increasing the current density to 125 A/ft2 for 5 min with the thought of increasing coverage and plating rate. Unfortunately, the rejection rate remained at 95%. The plating thickness was actually reduced, and the distribution was 216.74 ± 130.74 nm on the screw head and 32.84 ± 18.78 nm (at least some improvement there) on the threads. The results relate to the fact that a higher current density has reduced efficiency in the trivalent chromium process. There was no burning in high current density areas, but the overall lower plating rate was 43 μm/min.
After many trials varying different operating parameters - current density, pH, temperature - we concluded that the electrolyte itself needed to be modified to improve coverage.
Electrolyte modification
The venerable Hull cell was used to study changes in a proprietary bath formulation to improve the coverage. The basic chloride/sulfate-based electrolyte was retained, with its superior performance in plating speed, thickness build-up and corrosion resistance with known rack plating experience.
At this point, we decreased the rejects form over 95% to less than 33% rejects in the barrel process. The thickness distribution was 259.02 ± 124.14 nm on the screw head and 94.06 ± 94.79 nm (at least some improvement there) on the threads. Although still far from success, we did achieve good parts with good thickness at a lower current density and with a fast plating rate of 51 μm/min with a surface color that was identical to that from rack plating processes. This thickness is typical of exterior automotive high performance chromium. This was a major breakthrough that we could achieve a barrel trivalent chromium process.
Barrel contact area modification
During the research we also tried modifying the contact area in the barrel. We did this by simply exposing more area on the internal danglers. After the change, we tried a lower current density of 50 A/ft2 in the modified trivalent solution, and at 15% rejects, had fewer rejects than previous runs at higher current density. The thickness distribution was 217.69 ± 107.95 nm on the screw head and 83.20 ± 63.34 nm on the threads.
We then increased the current density to 80 A/ft2 and had approximately 5-10% rejects. The thickness distribution was 356.63 ± 143.22 nm on the screw head and 93.87 ± 56.96 nm on the threads.
Mixed load study
When we first started this project, we decided to plate difficult parts, i.e., parts with sharp points to rob current, as well as low current density areas such as the recesses on the head and deep threads of screw fasteners. We the mixed the load of screws with some tool parts and we had no rejects, even at 70 A/ft2 and with good thickness with only 5 min of plating time. The thickness distribution was 181.41 ± 63.38 nm on the screw head and 104.29 ± 60.52 nm on the threads.
Summary and conclusion
The data in the study is summarized in Table 1. By modifying the electrolyte and optimizing the cathode contact area, barrel trivalent chromium is a commercially viable process.
Table 1 - Summary of plate distribution results in the decorative trivalent chromium barrel plating study. Plating time = 5 min.
Future work
Our future plans involve:
- Identification of additional trial sites
- Evaluation in larger barrel sizes
- Evaluation on other part geometries
- Completion of field testing and analysis
About the author

Mark Schario is Executive Vice-President at Columbia Chemical Corporation, headquartered in Brunswick, Ohio. He joined Columbia Chemical in 2010 as Vice President, Technologies and now serves as Vice President, Global Business Development for the company. He has over 30 years of experience in the surface finishing industry. Mark also functions as the company’s top liaison to the automotive industry and is Committee Chairman to ASTM B08 on Metallic and Inorganic Coatings which has jurisdiction over 132 standards. He is a member of the NASF and has earned the industry designation of CEF/Certified Electroplater-Finisher. Mark holds an Executive MBA from the Weatherhead School of Management at Case Western Reserve University.
*Compiled by Dr. James H. Lindsay, Technical Editor - NASF
** Corresponding author:
Mark Schario
Executive Vice President
Columbia Chemical Corporation
1000 Western Drive
Brunswick, OH 44212
Phone: 440-840-7166
Email: mark.schario@columbiachemical.com
Related Content
Troubleshooting Alkaline Zinc
One of the most common problems that can arise when plating with alkaline zinc is an imbalance of brightener in the solution. In this helpful Ask the Expert article, Chad Murphy of Columbia Chemical discusses how different zinc metal concentrations and brightener concentrations can impact efficiency.
Read MoreInnovation in Plating on Plastic
Plating on advanced plastics solution offers improved adhesion, temperature resistance and cost savings.
Read MoreProducts Finishing Reveals 2024 Qualifying Top Shops
PF reveals the qualifying shops in its annual Top Shops Benchmarking Survey — a program designed to offer shops insights into their overall performance in the industry.
Read MoreAn Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read More











.jpg;maxWidth=300;quality=90)









