Electro-codeposition of MCrAlY Coatings for Advanced Gas Turbine Applications - 4th Quarterly Research Report
This NASF-AESF Foundation research project report covers the fourth quarter of project work (October-December 2018) on this AESF Foundation Research project at the Tennessee Technological University.
Editor’s Note: This NASF-AESF Foundation research project report covers the fourth quarter of project work (October-December 2018) on an AESF Foundation Research project at the Tennessee Technological University, Cookeville, Tennessee. A printable PDF version of this report is available by clicking HERE.
Summary: In this quarter, we focused on the effect of the concentration of CrAlY-based particles in the plating solution (particle loading) on the particle incorporation in the electro-codeposited coatings. For MCrAlY powder, when the particle concentration in the plating solution was increased from 10 to 30 g/L, the particle incorporation remained around 50 vol%. For CrAlY(Ta) powder, an increase of the particle incorporation was observed for 20 mA/cm2 as the particle loading was increased from 20 to 40 g/L, while further increase of particle loading from 40 to 60 g/L did not increase the particle incorporation significantly. At lower current density levels (e.g., 10-15 mA/cm2), the effect of particle loading was minimal. The trend observed in the current study was in good agreement with previous findings in the literature and it is consistent with the Langmuir adsorption isotherm on the electrode surface.
Technical report
I. Introduction
To improve high-temperature oxidation and corrosion resistance of critical superalloy components in gas turbine engines, metallic coatings such as diffusion aluminides or MCrAlY overlays (where M = Ni, Co or Ni+Co) have been employed, which form a protective oxide scale during service.1 The state-of-the-art techniques for depositing MCrAlY coatings include electron beam-physical vapor deposition (EB-PVD) and thermal spray processes.1 Despite the flexibility they permit, these techniques remain line-of-sight which can be a real drawback for depositing coatings on complex-shaped components. Further, high costs are involved with of the EB-PVD process.2 Several alternative methods of making MCrAlY coatings have been reported in the literature, among which electro-codeposition appears to be a more promising coating process.
Electrolytic codeposition (also called “composite electroplating”) is a process in which fine powders dispersed in an electroplating solution are codeposited with the metal onto the cathode (specimen) to form a multiphase composite coating.3,4 The process for fabrication of MCrAlY coatings involves two steps. In the first step, pre-alloyed particles containing elements such as chromium, aluminum and yttrium are codeposited with the metal matrix of nickel, cobalt or (Ni,Co) to form a (Ni,Co)-CrAlY composite coating. In the second step, a diffusion heat treatment is applied to convert the composite coating to the desired MCrAlY coating microstructure with multiple phases of β-NiAl, γ-Ni, etc.5
Compared to conventional electroplating, electro-codeposition is a more complicated process because of the particle involvement in metal deposition. It is generally believed that five consecutive steps are engaged:3,4 (i) formation of charged particles due to ions and surfactants adsorbed on particle surface, (ii) physical transport of particles through a convection layer, (iii) diffusion through a hydrodynamic boundary layer, (iv) migration through an electrical double layer and finally, (v) adsorption at the cathode where the particles are entrapped within the metal deposit. The quality of the electro-codeposited coatings depends upon many interrelated parameters, including the type of electrolyte, current density, pH, concentration of particles in the plating solution (particle loading), particle characteristics (composition, surface charge, shape, size), hydrodynamics inside the electroplating cell, cathode (specimen) position, and post-deposition heat treatment if necessary.3-6
There are several factors that can significantly affect the oxidation and corrosion performance of the electrodeposited MCrAlY coatings, including: (i) the volume percentage of the CrAlY powder in the as-deposited composite coating, (ii) the CrAlY particle size/distribution, and (iii) the sulfur level introduced into the coating from the electroplating solution. This three-year project aims to optimize the electro-codeposition process for improved oxidation/corrosion performance of the MCrAlY coatings. The three main tasks are as follows:
- Task 1 (Year 1): Effects of current density and particle loading on CrAlY particle incorporation.
- Task 2 (Year 2): Effect of CrAlY particle size on CrAlY particle incorporation.
- Task 3 (Year 3): Effect of electroplating solution on the coating sulfur level.
In this reporting period, we focused on the the effect of the CrAlY-based particle concentration in the plating solution (particle loading) on the particle incorporation in resultant composite coatings.
II. Experimental procedure
2.1. Substrate alloys and powders
Substrates were made from available nickel-based alloys including Ni 200 (>99.0 Ni, with 0.25 Cu-0.40 Fe-0.35 Mn-0.15 C-0.35 Si-0.01 S max., in wt%) and René 80 (Ni-3.0 Al-14.1 Cr-9.7 Co-4.3 W-4.0 Mo-5.0 Ti-0.18 C in wt%, 130B-200 Zr-7 S in ppmw). Disc specimens (1.6 mm thick, ~17 mm in diameter) were cut with an abrasive cutting saw. The specimens were ground to #600 grit using SiC grinding papers, followed by grit blasting with #220 Al2O3 grit, and were then ultrasonically cleaned in hot water and acetone. Laboratory ball-milled CrAlYTa powder and commercial gas-atomized MCrAlY (CoNiCrAlY) powder were used in the electro-codeposition experiments. The as-received MCrAlY powder had a particle size in a range of 4 to 150 µm and was sieved through a 20 µm sieve. The chemical compositions and other characteristics of the two powders are given in Table 1.
Table 1 - Chemical compositions (wt%) and other characteristics of the two alloy powders used in the electro-codeposition experiments.
|
|
Co |
Ni |
Cr |
Al |
Y |
Ta |
D50 (μm) |
Shape |
Density (g/cm3) |
|
Atomized MCrAlY (PSI) |
Bal. |
32.0 |
21.0 |
8.0 |
0.8 |
— |
12.5 |
Spherical |
7.5 |
|
Ball-milled CrAlYTa (TTU) |
— |
— |
60.6 |
25.3 |
1.5 |
12.6 |
— |
Irregular |
5.5 |
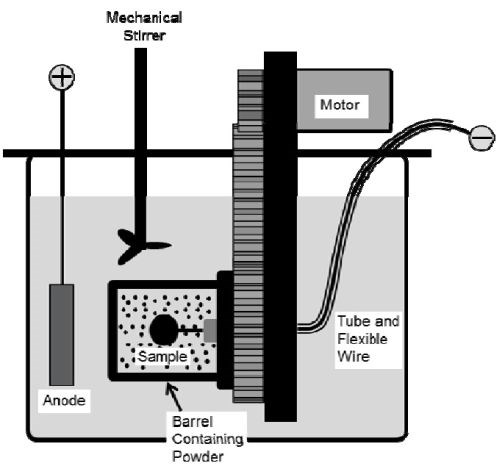
Figure 1 - Schematic of the barrel system.
2.2. Electro-codeposition
A rotating barrel system shown in Fig. 1 was employed in the electro-codeposition experiments; details can be found in the 2018-Q1 Report. Watts nickel plating solution was used and the nickel anode was placed outside of the barrel along with a mechanical stirrer and heating coil. The specimens were plated at 50°C with a pH level of 3.7-3.9. The powder concentration in the plating solution was varied from 10 to 60 g/L and the barrel rotation speed was kept at 7 RPM. For the CrAlYTa powder, three current density levels 10, 15, and 20 mA/cm2 were employed, while for the MCrAlY powder the electro-codeposition experiments were only carried out using a current density of 20 mA/cm2.
2.3. Coating characterization
The Ni-CrAlY(Ta) composite coatings were characterized using scanning electron microscopy (SEM) equipped with energy dispersive spectroscopy. Prior to metallographic sample preparation, the specimens were copper-plated to improve the edge retention. To determine the volume fraction of the incorporated CrAlY particles, multiple backscattered electron images were taken from different locations along the coating cross-section, which were then processed using the ImageJ software. The brightness and contrast of the image were adjusted by setting a proper threshold such that the particles were separated from the background. The area fraction of the CrAlY particles was determined, which was assumed equivalent to its volume fraction.
III. Results and discussion
The as-deposited coatings consisted of CrAlYTa or MCrAlY particles and a nickel matrix. Typical coating microstructures were presented in previous quarterly reports. Figure 2 shows the particle incorporation in these metal matrix composite coatings as a function of the particle loading. For the MCrAlY powder, when the particle concentration in the plating solution was increased from 10 to 30 g/L, the particle incorporation remained around 50 vol%. For the CrAlYTa powder, an increase of the particle incorporation was observed for 20 mA/cm2 as the particle loading was increased from 20 to 40 g/L, while a further increase of particle loading from 40 to 60 g/L did not lead to a drastic increase in particle incorporation. At the lower current density levels (e.g., 10-15 mA/cm2), the effect of particle loading was not evident for the CrAlYTa powder.
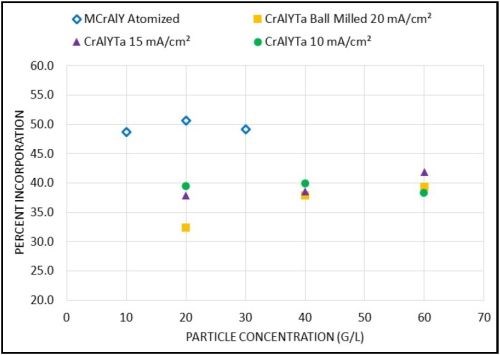
Figure 2 - Effect of particle concentration on particle incorporation in the as-deposited coatings for commercial atomized MCrAlY and laboratory ball-milled CrAlYTa powders.
Particle loading is one of the important factors affecting the particle incorporation in electro-codeposited composite coatings. Several studies have shown that when the particle concentration in the plating solution is low, increases in particle concentration lead to substantial increases in particle incorporation in the coating.7-11 However, as the particle loading increases, the increase in particle incorporation begins to plateau. Such a tendency is consistent with the Langmuir adsorption isotherm on the electrode surface.12 For plating solutions with a low particle concentration, the particle incorporation is limited by the supply of particles to the cathode (i.e., the specimen) surface by agitation and diffusion. Increasing the overall amount of particles in the solution enhances the probability of particles of reaching the electrochemical double layer at the cathode and thus improves particle incorporation. However, hydrodynamic effects due to particle-particle interactions become more dominant at high particle concentrations, and thus have a negative impact on particle incorporation.
Although this trend has been confirmed for a wide range of metal particle systems, the threshold particle concentration at which saturation (the plateau) occurs depends on the powder characteristics and the electro-codeposition conditions.13 Susan10 studied Ni-Al composite coatings containing aluminum particles (1.5-3.5 μm size) embedded in a nickel matrix electrodeposited in a vertical arrangement in a beaker, and observed a plateau at 300-400 g/L. In a later study carried out by Liu11 using a horizontal sedimentation configuration, the plateau occurred at ~40 g/L. In our current study, for gas-atomized MCrAlY particles with a higher density (7.5 g/cm3) and larger particle size (12.5 m), the plateau started at a lower particle concentration (10 g/L) with a plating current density of 20 mA/cm2. For ball-milled CrAlYTa powder with a reduced density (5.5 g/cm3) and particle size (5.6m), the plateau was observed at ≥40 g/L at 20 mA/cm2, whereas the plateau started at 20 g/L with 10-15 mA/cm2 current densities. The overall trend, however, was in good agreement with previous findings in the literature.
IV. Future work
In the next quarter (1st quarter in Year 2), CrAlY powders of different particle sizes will be utilized to assess the effect of particle size on the CrAlY particle incorporation in NiCo-CrAlY composite coatings.
References
1. G.W. Goward, Surf. Coat. Technol., 108-109, 73-79 (1998).
2. A. Feuerstein, et al., J. Therm. Spray Technol., 17 (2), 199-213 (2008).
3. C.T.J. Low, R.G.A. Wills and F.C. Walsh, Surf. Coat. Technol., 201 (1-2), 371-383 (2006).
4. F.C. Walsh and C. Ponce de Leon, Trans. Inst. Metal Fin., 92 (2), 83-98 (2014).
5. Y. Zhang, JOM, 67 (11), 2599-2607 (2015).
6. B.L. Bates, J.C. Witman and Y. Zhang, Mater. Manuf. Process, 31 (9), 1232-1237 (2016).
7. A. Hovestad, R.J.C.H.L. Heesen and L.J.J. Janssen, J. Appl. Electrochem., 29 (3), 331-338 (1999).
8. J. Foster, B.P. Cameron and J.A. Carew, Trans. Inst. Met. Finish., 63 (3-4), 115-119 (1985).
9. H. Liu and W. Chen, Surf. Coat. Technol., 191 (2-3), 341-350 (2005).
10. D.F. Susan, “Diffusion and High-Temperature Oxidation of Nickel-Aluminum-based Composite Coatings,” Ph.D. Dissertation, Lehigh University, 1999.
11. H. Liu, “Electrodeposited Ni3Al Base Intermetallic Coatings and Their Resistance to High Temperature Degradation in Hydrocarbon Cracking Environments,” Ph.D. Dissertation, University of Alberta, 2006.
12. N. Guglielmi, J. Electrochem. Soc., 119 (8), 1009-1012 (1972).
13. A. Hovestad and L.J.J. Janssen, “Electroplating of Metal Matrix Composites by Codeposition of Suspended Particles,” in Modern Aspects of Electrochemistry, B.E. Conway, C.G. Vayenas, R.E. White, and M.E. Gamboa-Adelco, Eds. Springer, Boston, MA (2005); pp. 475-532.
Past project reports
1. Quarter 1 (January-March 2018): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 82 (12), 13 (September 2018); Full paper: http://short.pfonline.com/NASF18Sep1.
2. Quarter 2 (April-June 2018): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 83 (1), 13 (October 2018); Full paper: http://short.pfonline.com/NASF18Oct1.
2. Quarter 3 (July-September 2018): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 83 (3), 15 (December 2018); Full paper: http://short.pfonline.com/NASF18Dec1.
About the authors

Dr. Ying Zhang is Professor of Mechanical Engineering at Tennessee Technological University, in Cookeville, Tennessee. She holds a B.S. in Physical Metallurgy from Yanshan University (China)(1990), an M.S. in Materials Science and Engineering from Shanghai University (China)(1993) and a Ph.D. in Materials Science and Engineering from the University of Tennessee (Knoxville)(1998). Her research interests are related to high-temperature protective coatings for gas turbine engine applications; materials synthesis via chemical vapor deposition, pack cementation and electrodeposition, and high-temperature oxidation and corrosion. She is the author of numerous papers in materials science and has mentored several Graduate and Post-Graduate scholars.
Jason C. Whitman is a Ph.D. student in the Department of Mechanical Engineering, Tennessee Technological University, in Cookeville, Tennessee. His primary work is involved with the AESF Foundation Research Project R-119, Electro-codeposition of MCrAlY Coatings for Advanced Gas Turbine Applications.
*Corresponding author:
Dr. Ying Zhang, Professor
Department of Mechanical Engineering
Tennessee Technological University
Cookeville, TN 38505-0001
Tel: (931) 372-3265
Fax: (931) 372-6340
Email: yzhang@tntech.edu
Related Content
AESF Heritage: SUR/FIN 2000 – European Academy of Surface Technology: Copper Microelectrodeposition: The Influence of Different Additives on Growth in Microprofiles
In this work, a pH 3.0, 0.8M copper sulfate electrolyte was used for plating onto both blanket and patterned silicon wafers. This novel electrolyte, proposed by U. Landau, involves a number of beneficial effects with respect to the conventional bath.
Read MoreCalculating Applied Media Force During Vibratory Finishing
What appear to be identically set-up vibratory bowls will finish identical loads of parts in varying time cycles. This paper offers a new technique to better predict what the operator will produce, by measuring the force applied to the parts. It is the efficiency of that force which controls the efficiency and speed of the refinement cycle.
Read MoreMaterial Database Enables Coating Thickness Measurement Without Calibration
The database from Coatmaster AG has calibrations of over 400 different RAL colors.
Read MoreRead Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More



















