Electrolytic hard chromium plating (EHC) is a critical surface finishing technology that is used for applying functional coatings for corrosion and wear resistance to aircraft components in manufacturing operations and for re-build of worn or corroded components. However, EHC plating baths contain hexavalent chromium, which is a known carcinogen and environmental hazard. Therefore, the replacement of EHC in aircraft manufacturing activities and maintenance depots is a high priority for the U.S. Department of Defense (DoD).
Nanocrystalline cobalt-phosphorus plating (nCoP) is commercially available as an environmentally compliant alternative to EHC coatings. As an electrodeposition process, nCoP is fully compatible with the existing EHC infrastructure, but exhibits higher cathodic efficiencies and deposition rates than EHC, thus yielding higher throughput, reduced facility footprint and reduced energy consumption. Further, nCoP offers significant performance enhancements over EHC including superior sliding wear, enhanced lubricity and corrosion resistance, and much improved fatigue properties.
nCoP was developed in participation with the DoD’s Strategic Environmental Research and Development Program (SERDP) and Environmental Security Technology Certification Program (ESTCP). A currently ongoing ESTCP program (WP-0936) along with leveraged support from the Navy's Environmental Sustainability Development to Integration (NESDI) program (Project #348) aims at fully qualifying nCoP through performance testing and demonstration/validation on a number of components from NAVAIR (air vehicle and ground support equipment) and NAVSEA (shipboard machinery components and ground support equipment).
A general overview of the process and properties of nCoP coatings are presented in comparison to EHC with recent supporting data. A case study of one application area for nCoP is showcased.
Keywords: Hexavalent chromium replacement, hard chromium replacement, nanocrystalline cobalt-phosphorus, electrodeposited Co-P
Introduction
The replacement of electrolytic hard chromium (EHC) plating in aircraft manufacturing activities and maintenance depots is a high priority for the U.S. Department of Defense (DoD). Hard chromium plating is a technique that has been in commercial production for over 50 years and is a critical process that is utilized both for applying hard coatings to a variety of aircraft components in manufacturing operations and for general repair of worn or corroded components during maintenance. Chromium plating baths contain hexavalent chromium (Cr+6), a known carcinogen and environmental hazard having a level of toxicity greater than arsenic or cadmium. During operation, chromium plating tanks emit a Cr+6 mist into the air, which must be ducted away and removed by scrubbers. Wastes generated from plating operations must be disposed of as hazardous waste and plating operations must abide by Environmental Protection Agency (EPA) emissions standards and Occupational Safety and Health Administration (OSHA) permissible exposure limits (PEL).
Drivers for change
By far the largest regulatory cost driver for EHC plating has been the reduction of the Cr+6 PEL from 52 µg/m3 to 5 µg/m3 by OSHA in 2006. This rule also included provisions for employee protection such as preferred methods for controlling exposure, respiratory protection, protective work clothing and equipment, hygiene areas and practices, medical surveillance, hazard communication and record-keeping. The expected one-time compliance costs, as determined by OSHA, in all industries including electroplating, welding, painting and chromate production, was $226 million, although the surface finishing industry expected that the costs would be substantially higher. There would also be increased annual recurring costs associated with health monitoring, record-keeping, etc. Although gaseous emissions from EHC plating operations must also conform to the National Emissions Standards for Hazardous Air Pollutants (NESHAPS) and any solid or liquid waste generated from EHC (such as plating sludge, lead chromate from decomposed anodes, precipitates from barium treatments for sulfate control, etc.) must be disposed of as hazardous waste in accordance with Resource Conservation and Recovery Act (RCRA) regulations, the costs associated with compliance with these regulations are minor compared to the cost of compliance with the Cr+6 PEL reduction.
In addition to the national regulatory rules, on 9 April 2009, a memo from the Office of the Secretary of Defense (OSD) was released to mitigate more aggressively the unique risks to DoD operations now posed by Cr+6 as a result of increased international and national restrictions. The memo instructs DoD Military Departments to restrict the use of Cr+6 unless no cost-effective alternatives are identified. Furthermore, this would force adoption of Cr+6-free coatings and production methods unless otherwise approved directly by the Program Executive Office (PEO) or equivalent level, in coordination with the Military Department's Corrosion Control and Prevention Executive (CCPE), to certify there is no acceptable alternative to the use of Cr+6 on a new system. Based on the projections of the metal finishing industry and internal assessments, it was clear that a reduction of the Cr+6 PEL would greatly increase the cost and processing times associated with EHC plating within DoD.
Existing alternatives to EHC
Previous research and development efforts1,2 had established that high-velocity oxygen-fuel (HVOF) thermal spray coatings are the leading candidates for replacement of EHC. Using commercially available systems, HVOF thermal spraying can be used to deposit both metal alloy and ceramic/metal (e.g., WC/Co) coatings that are dense and highly adherent to the base material. They also can be applied to thicknesses in the same range as those currently being used for EHC plating. Extensive materials testing generally showed that HVOF coatings such as WC/17Co demonstrate performance superior in fatigue, wear and corrosion to EHC coatings. Rig and flight tests on WC/17Co-coated components showed acceptable performance for the HVOF coatings and, in many cases, superior performance to what would be expected had the components been coated with EHC. As a result of these projects, HVOF is being implemented at a number of Air Force and Navy repair facilities for processing of landing gear, propeller hub and gas turbine engine components.
However, HVOF is a line-of-sight (LOS) process and is therefore limited in its application to recessed or internal diameters on parts. Evaluations of the application of EHC on many different types of aircraft components at DoD repair depots showed that between 20-25% of all EHC applications were not amenable to HVOF. With the ultimate goal of eliminating all EHC plating from DoD operations, the DoD’s Strategic Environmental Research and Development Program (SERDP) sponsored several projects to investigate alternative technologies that would be capable of replacing EHC on all types of geometries including internal surfaces. The projects were completed in late 2003 and an assessment of the results from the projects indicated that electroplated nanocrystalline cobalt-phosphorus (nCoP) had the broadest capability for replacing EHC on all types of components and that it was the most ready for demonstration/validation.
The nCoP alloys were produced using an electrodeposition process similar to EHC. This allows for the retention of numerous benefits associated with hard chromium coating technology (i.e., non-line-of-sight application, excellent coating adhesion, dimensional consistency and superior surface finish) and leverages existing EHC plating infrastructure. Further, the nCoP alloys exhibited material properties in many cases superior to EHC. As a result, nCoP is currently being demonstrated and validated for use as an EHC alternative in DoD repair and overhaul depots with support from the US DoD’s Environmental Security Technology Certification Program (ESTCP) and the Canadian Strategic Aerospace and Defense Initiative (SADI).
nCoP as an EHC alternative
nCoP is an electrodeposited nanocrystalline cobalt-phosphorus alloy that exhibits properties that are equivalent to (and in many ways better than) EHC. As described in subsequent sections, nCoP is an alternative to EHC processes on both LOS and non-line-of-sight (NLOS) surfaces, and can be viewed as part of an overall strategy to replace the currently used EHC processes and eliminate environmental and worker safety issues while significantly improving performance and reducing life-cycle costs.
Production process
The nCoP process offers significant improvements over EHC, as summarized in Table 1. Like EHC, nCoP is produced by electrodeposition,3-6 and therefore represents a drop-in alternative technology that is fully compatible with the current EHC electroplating infrastructure, and renders nCoP well-suited for application to both LOS and NLOS surfaces. Unlike EHC, the nCoP process uses no constituents on EPA or AFMC lists of hazardous materials, nor does it generate hazardous emissions or by-products.
Significant reductions in energy consumption and increases in throughput can be achieved with the nCoP process. The overall plating efficiency of the nCoP process is greater than 90%, compared to less than 35% for EHC. Further nCoP has a high deposition rate, ranging from 0.003-0.004 in./hr, depending on current density, in contrast to EHC which typically plates at 0.0005-0.0010 in./hr.
Table 1 - Comparison of nCoP and EHC processes.
Properties
As summarized in Table 2 and described in detail below, the properties of nCoP are equivalent to, and in many ways better, than EHC.
Table 2 - Comparison of nCoP and EHC properties.
| nCoP | EHC |
Appearance | Pit/pore/crack-free | Microcracked |
Microstructure | Nanocrystalline | --- |
Hardness | As-deposited | 530-600 VHN | 600 VHN Min. |
Heat-treated | 750 VHN | --- |
Sliding wear | Wear volume loss | 6 - 7×10-6 mm3/Nm | 9 - 11×10-6 mm3/Nm |
Coefficient of friction | 0.4 - 0.5 | 0.7 |
Pin wear | Mild | Severe |
Corrosion resistance (1,000 hr salt spray) | Protection rating 8 | Protection rating 2 |
Hydrogen embrittlement | Pass with bake | Pass with bake |
Fatigue | Credit vs. EHC | Significant debit vs. bare |
Appearance and Microstructure: Visually, nCoP coatings are uniformly smooth and shiny, similar to EHC. Microscopically, nCoP is a fully dense structure, free from pits, pores and microcracks as shown in Fig. 1. The nCoP exhibits a hexagonal close-packed (HCP) crystal structure, which is the equilibrium structure typically found in conventional cobalt at room temperature. Unlike conventional cobalt, however, nCoP exhibits a nanocrystalline microstructure, with an average grain size in the range of 5 to 15 nm. An average grain size in this range gave the optimum combination of strength and ductility.
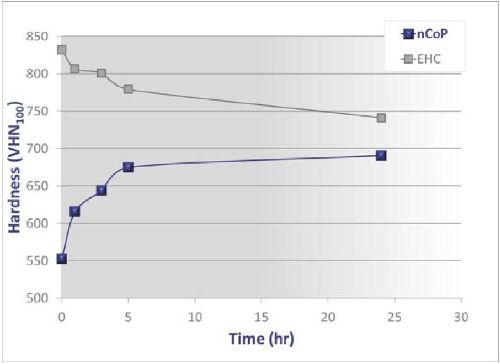
Hardness: The nCoP coatings display significant increases in hardness and strength relative to coarser-grained, conventional cobalt. Through Hall-Petch strengthening and a solid solution hardening mechanism, microhardness values range from 530 to 600 VHN. A further increase in hardness of up to 750 VHN can be obtained by precipitation hardening the as-deposited material.7 Increases in hardness are seen at relatively low temperatures, such as those typically used for hydrogen embrittlement relief baking of high strength steels after plating processes (375°F). As shown in Fig. 2, the microhardness of nCoP increases by up to 150 VHN following such a heat treatment. Interestingly, under these same heat treatment conditions, the microhardness of EHC decreases by 100 VHN. This indicates that although EHC has a higher hardness than nCoP in the as-deposited state, their hardnesses converge at elevated temperatures including the hydrogen embrittlement relief baking employed regularly.
Wear resistance and lubricity: Pin-on-disc sliding wear testing indicates that nCoP exhibits less wear loss than EHC. Further, the wear loss of the mating material is significantly less severe. The nCoP has a lower coefficient of friction than EHC, 0.4 and 0.7 respectively, resulting in enhanced lubricity.
Hydrogen embrittlement: The high plating efficiency of the nCoP process leads to significantly less hydrogen generation at the cathode compared to the EHC process, thus minimizing the likelihood of hydrogen uptake and subsequent embrittlement of susceptible materials (i.e., high-strength steels). Hydrogen embrittlement tests conducted in accordance with ASTM F519 using Type 1.a1 4340 steel specimens (loaded for 200 hr at 75% notch fracture strength) indicate that the standard hydrogen embrittlement relief baking procedures for EHC can be applied to the nCoP to fully eliminate the risk of embrittlement.
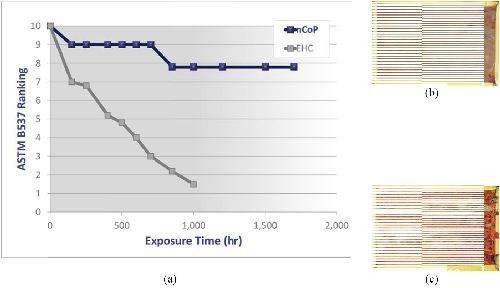
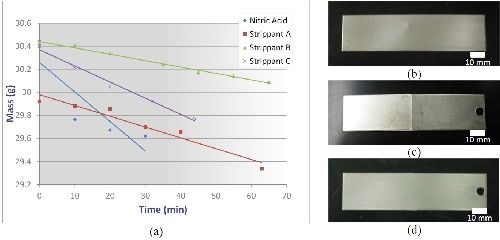
Corrosion resistance: Figure 3(a) shows the ASTM B537 protection rating as a function of exposure time for nCoP-coated (0.002 in.) and EHC-coated (0.004 in.) low alloy steel after being exposed to a salt spray environment per ASTM B117. The nCoP performed very well, decreasing to only a protection/appearance rating of 8 after 1000 hr exposure time, compared to a rating of less 2 for EHC after the same exposure time.9 Indeed, as shown in Fig. 3, images of nCoP-coated and EHC-coated mild steel following 48 hr salt spray indicate that red rust formed on EHC-coated samples but not on nCoP-coated samples. Surface composition analysis conducted by energy dispersive x-ray spectroscopy confirmed the presence of iron species on the EHC-coated sample, while only cobalt was found on the nCoP-coated sample. This underscores the superior corrosion protection of nCoP compared to EHC.
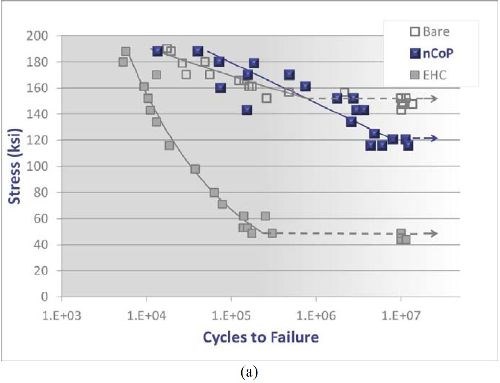
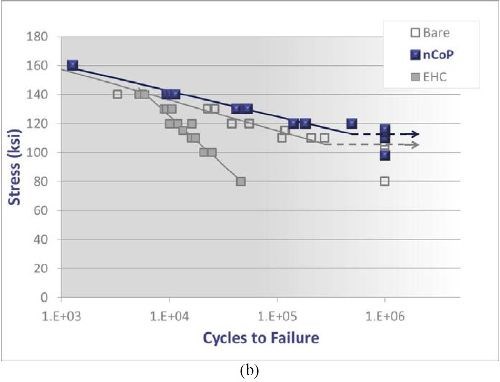
Fatigue resistance: Rotating beam and axial fatigue testing were conducted on nCoP- and EHC-coated 4340 steel. As shown in the S-N curve in Fig. 4(a) for substrate material hardened to 260-280 ksi UTS, EHC exhibits a significant fatigue debit for EHC coating compared to the bare material. Alternatively, the nCoP coating shows a fatigue life similar to the bare material at most loads, albeit at the lowest applied loads, it shows some debit compared to the bare material. However, this is not nearly as severe as the debit exhibited by the EHC coated material. At all loads, nCoP exhibits significantly enhanced fatigue performance compared to EHC. Further, as shown in Fig. 4(b) for slightly softer substrate material (180-200 ksi UTS), axial fatigue testing indicates that nCoP exhibits a fatigue credit with respect to EHC and the bare material.5
Technology demonstration and evaluation
Demonstration site
The Fleet Readiness Center Southeast (FRCSE) in Jacksonville, Florida was selected as the site for demonstration of the nCoP plating technology for tank batch plating. FRCSE has been the most proactive of the Navy depots in implementing HVOF thermal spray coatings for hard chromium replacement. Through implementation of HVOF and other activities, the depot recently was able to shut down three 2000-gallon chromium plating tanks. The depot does, however, still have a significant chromium plating facility with several tanks of size ranging from 200 to 2000 gallons.
Utilizing existing infrastructure, a small process line was modified for the purpose of demonstrating nCoP in the depot environment, and has been in operation since 2006. The line consists of an activation tank, the nCoP plating tank and associated rinse tanks (Fig. 5). All prior processing steps, i.e. cleaning, blasting, masking and racking, are performed within the general area of plating facility. This allows for the treatment of a large range of substrate materials encountered by the depot. Processing of high strength low alloy steels has been demonstrated to date and demonstrations for stainless steels, aluminum alloys and nickel alloys are planned for the near future.
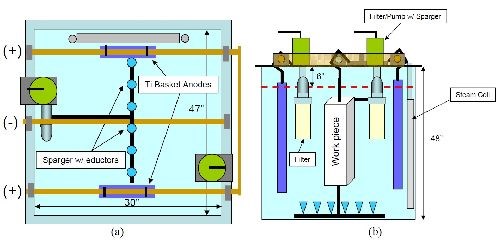
An existing process tank formerly used for EHC plating was dedicated to the nCoP process. The tank has a capacity of approximately 300 gallons and was equipped with a drop-in liner made of a modified vinyl based terpolymer, 0.125 in. thick. The tank is equipped to process parts within a 20 × 20 × 36 in. envelope. Other installed equipment includes a steam coil, steam line, regulator, in-tank pump and filter using 3-µm size polypropylene cartridges. A sparger equipped with eductors, nickel plated copper bus bars and a level sensor for maintaining the solution level was also installed. Titanium anode mesh baskets with anode bags were also installed to contain the consumable cobalt anode pieces. The pulsing power supply is a 500 A average/1500 A peak current unit that was fully evaluated by Integran prior to shipment to FRCSE. Figure 6 illustrates the installed nCoP process tank at FRCSE, and Fig. 7 illustrates schematics of the plating tank design and layout.
Supporting processes
As is also required for other coating processes, pre- and post-plating treatments are required for the nCoP process. Substrate masking and coating removal processes have been validated, and coating grinding and substrate activation processes are currently under evaluation.
Prior to plating, parts will need to be free of existing deposits. Removal of previous coatings may be performed by chemically stripping in special solutions or by mechanical grinding and/or other removal methods. Strippants for removal of nCoP from ferrous substrates were evaluated and selected. These consisted of three nitro-organic oxidizers with amino compounds, which were compared to a baseline of nitric acid. To assess coating removal rates in these solutions, mild steel coupons plated with nCoP were immersed in each of the solutions and their mass recorded at regular time intervals until the coating was completely removed. Coating removal was verified by visual examination, weight loss and elemental surface analysis. Stripped samples were then visually and microscopically examined for signs of substrate damage. As shown in Fig. 8(a), the rate of removal was found to be 0.001 to 0.004 in./hr. While all strippants were found to completely remove nCoP without damaging the substrate, Strippant C provided the highest stripping rate and was selected as the preferred strippant solution of those evaluated. In addition, a strippant solution used to remove electroless nickel was also successfully tested at FRCSE. Its coating removal rates are also expected to range between 0.001 to 0.004 in./hr.
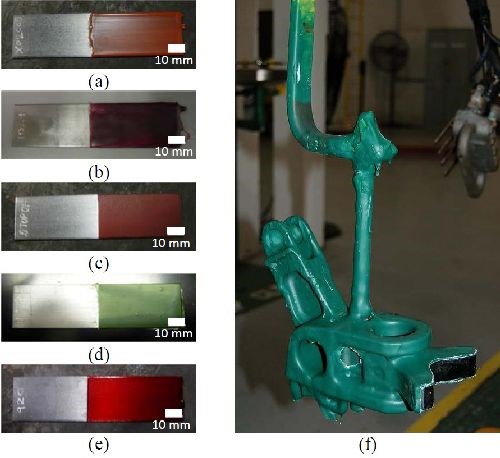
When selective areas of components need to be plated, other areas on the part must be masked for compliance. To protect these areas properly, suitable maskants able to withstand the chemicals to which they will be exposed must be used. In addition, these maskants must be reasonably easy to apply and remove from the substrates. As such, five solvent-based maskants, shown in Figs. 9(a)-(e), were evaluated for their ability to prevent unwanted areas from being plated and for their effects on electrolyte and deposit quality. One of the five solvent-based maskants evaluated [Fig. 9(d)] was selected for use at FRCSE. Figure 9(f) shows a photo of a T-45 pivot assembly racked and masked with the selected maskant.
Case study: T-45 arresting hook pivot assembly
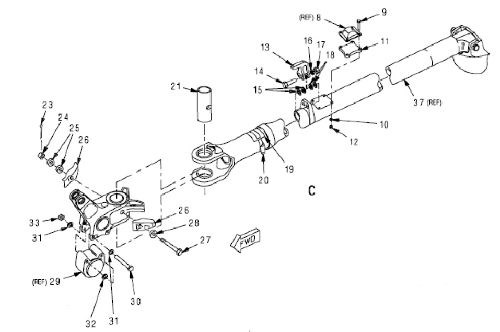
A T-45 arresting hook pivot assembly is one of the components that will be utilized to evaluate field performance of the applied nCoP coating through operational flight testing of a U.S. Navy T-45 trainer aircraft, shown in Fig. 10. The arresting hook assembly is a pylon mounted, fixed pivot configuration which is used for cable arrestments. The hook assembly attaches to the shank assembly and engages the arresting cable during cable arrested landings. The cylinder assembly is a self-contained hydraulic pneumatic actuator, preloaded in the bore of the shank assembly. The cylinder damper interfaces with the roller housing assembly and together they contact the surface cams of the pivot assembly to dampen horizontal movement of the arresting hook assembly. The cylinder assembly aids in maintaining the central position of the arresting hook assembly and restores that position during lateral displacement caused by arrestments. Figure 11 illustrates a schematic diagram of the arresting gear configuration and arresting hook assembly.
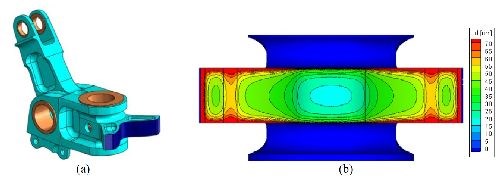
The surface cams of the pivot assembly, as shown in Fig. 12, are the specific areas to be evaluated. These areas experience wear and are currently nickel coated and chromium capped. Due to their complex geometry, computational modeling of the coating quality (i.e., thickness, deposit property variations) was employed to assist with rack design (Elsyca Inc., Belgium). Chemical characterization of the plating solution was first conducted to provide input parameters for the model. The model was developed, and various rack designs were assessed for their impact on coating quality. An example of the model output for the thickness distribution from one rack design is shown in Fig. 13. The final rack design was selected based on model output, functionality and cost, and was used to produce parts as shown in Fig. 14. Parts were first stripped of residual EHC and nickel, cleaned, grit blasted, racked and masked. The racked part was then activated and plated, then deracked and demasked.
Future outlook
Property and performance testing is in progress to develop a comprehensive data set for the nCoP material. This consists of evaluations of coating quality, appearance, thickness, porosity, hardness, grain size, ductility, internal stress, adhesion, axial fatigue, corrosion, (ASTM B117, SO2 salt fog, beach exposure, open circuit potential), hydrogen embrittlement, hydrogen re-embrittlement, fluid compatibility, wear resistance (Taber abrasive, Falex block on ring, pin-on-disc, gravelometry, SATEC oscillating load, seal wear and leakage). Where necessary, nCoP performance will be benchmarked against EHC.
In addition, field testing of the T-45 pivot assembly will specifically address the wear and adhesion performance of an nCoP coating applied to the surface cams of the pivot assembly. The nCoP-coated pivot assembly and EHC-coated control will be installed on the aircraft and periodically inspected for deformation, cracks, corrosion and obvious wear during a mandatory off-aircraft inspection every 100 carrier arrestments. The number of arrestments and associated flight hours will be recorded to correlate performance to the component processed with traditional EHC. In addition to the T-45 component, other demonstration components include a spotting dolly lifting arm axel pin and a Marine Corps MK48 LVS (logistic vehicle system) hydraulic cylinder piston.
In parallel with performance testing, efforts to support technology transition to DoD depots is also underway. This consists of the validation of additional supporting processes, including substrate activation and coating grindability, development of local process specifications and industrial standards, and information dissemination to the other depots and stakeholders.
Conclusions
Nanocrystalline cobalt phosphorus (nCoP) is an environmentally-compliant alternative to electrolytic hard chromium (EHC) that is compatible with the existing EHC plating infrastructure and represents a drop-in replacement for EHC. As an electrodeposition process, nCoP can readily be applied to line-of-sight (LOS) and non-LOS surfaces. Due to its high process efficiency, nCoP exhibits higher plating rates and reduced energy consumption, resulting in increased throughput, reduced plant footprint and reduced cost compared to EHC. The nCoP is non-embrittling, and offers superior wear, corrosion and fatigue performance compared to EHC. As a result of its process and property enhancements, nCoP is currently being demonstrated and validated as a technically feasible and commercially viable replacement for EHC on multiple military systems for use in DoD repair and overhaul depots. In addition to DoD use, the nCoP process is currently in commercial use for the production of hydraulic cylinders for the fluid power market (TRL 9).10
Acknowledgements
The authors gratefully acknowledge funding from the U.S. DoD’s Environmental Security Technology Certification Program (project WP-0936) and the Canadian Strategic Aerospace and Defense Initiative (project 780-505205).
References
“High Velocity Oxy Fuel Final Results Report,” Final Report issued by Science Applications International Corporation under Government Contract F09603-90-D2215, Oklahoma City Air Logistics Center, Tinker Air Force Base, May 25, 1994.
“Hard Chrome Coatings - Advanced Technology for Waste Elimination,” Final Report under DARPA Grant Number NDA 972-93-1-0006, October 1997.
- U. Erb & A.M. El-Sherik, “Nanocrystalline Metals and Process of Producing the Same,” U.S. Patent 5,352,266 (1994).
- U. Erb, et al., “Nanocrystalline Metals,” U.S. Patent 5,433,797 (1995).
- D. Facchini, et al., “Electrodeposited Metallic-Materials Comprising Cobalt,” U.S. Patent Application No. US2010/0304182 (2010).
- Fine-grained metallic coatings having the coefficient of thermal expansion matched to the one of the substrate: G. Palumbo, et al., U.S. Patents 7,824,774 (2010); 7,320,832 (2008) and 7,910,224 (2011).
- D. Facchini, et al., Products Finishing, 73 (7), 14 (April, 2009).
- R.A. Prado, J. Benfer & D. Facchini, “Electrodeposition of Nanocrystalline Co-P Coatings as a Hard Chromium Alternative,” in Proc. ASETS Defense, Sustainable Surface Engineering for Aerospace & Defense, New Orleans LA, February 8-11, 2011; asetsdefense.org/documents/Workshops/SustainableSurfaceEngineering2011/44-Prado%20-%20ASETS_Defense_2011_final.pdf.
- B.D. Sartwell, P.M. Natishan, K.O. Legg, J.D. Schell & J.P. Sauer, Proc. AESF Plating Forum, March 1998, NASF Washington, DC, 1998.
- A. Moline, et al., “Industrial Implementation of Nanostructured Cobalt-Phosphorus Coatings at Enduro Industries LLC,” Proc. SUR/FIN 2011, Rosemont IL, June 13-15, 2011, NASF, Washington, DC, 2011.