Going Low-Temp
What to Consider when choosing ambient-temperature metal pretreatments
In recent years, metal finishers have been introduced to a variety of new technologies for pre-paint metal pretreatment systems, touting environmental benefits and energy cost savings. The new chemical treatment processes go by several generic names: nanoceramic, organo-silane, fluorozirconium, fluorotitanic, zirconization, zirconate, phosphonate or vanadium treatments, to name a few.
Obviously, they have various trade names in the marketplace, but for the benefit of the reader, this article will refer to them simply as “new pretreatments.”
These new pretreatments are designed to replace traditional metal pretreatments—a.k.a. iron phosphates. They’ve also been used to replace zinc phosphate processes in some applications. The two major benefits promoted by developers are the reduction of energy consumed in the chemical process and the reduction or elimination of phosphate discharge.
Sounds good, right? Yes, but one must carefully consider the practical application of implementing these new technologies. Since their introduction over the past several years, considerable field knowledge has been gained and there are many variables that need to be carefully examined before using these chemistries.
In general, use of these materials in commercial applications has presented some unique considerations. For instance, the new pretreatments are not as robust as the traditional pretreatments they replace, and they also react in a completely different fashion chemically.
To fully grasp how the chemistries can benefit your process, you need to have a full understanding of the chemical reaction that occurs when using these products. This will enable you to determine what products will be best for the substrates you finish, the type of paint you apply, the equipment you work with and your quality expectations.
This article will also review important process and substrate considerations—factors essential in ensuring that the new pretreatment you choose for your process will achieve desired results without disastrous problems.
Chemical Considerations
Iron and zinc phosphate products use phosphoric acid in their formulations. The phosphoric acid, combined with temperature and chemical accelerators, dissolves iron from the substrate being treated. In an iron phosphate process, an electro-chemical reaction occurs that converts the dissolved iron with the phosphate to form an iron phosphate conversion coating onto the substrate. In the case of zinc phosphate, zinc is primarily incorporated in this conversion coating.
Depending on the process parameters and the type of product used, the resulting conversion coating can be controlled to a desired thickness or weight level required for optimum performance. Weight of these coatings can be measured in mg/inch2 .
In both iron and zinc phosphate pretreatment processes, the reaction is not completely efficient. Much of the dissolved iron does not convert, instead forming a sludge in the process bath.
The majority of the new pretreatment technologies are formulated with fluorozirconic acid or fluorotitanic acid. These materials replace the phosphoric acid used in traditional iron or zinc phosphate formulations, thus making the new pretreatment product “phosphate-free.”
The fluoride part of these acids does not convert with iron from the substrate in the same manner as phosphoric acid in a phosphate pretreatment process. Instead, the fluoride aids in the formation of a non-reactive oxide surface film that inhibits further corrosion. This oxide layer is only a few atoms thick and is measured in nanometers. Oxide formation begins quickly on the substrate, and the oxide film thickness is not as important as the type of oxide coating formed.
If this oxidation process is not controlled properly, you will form an iron oxide (rust) coating. However, when oxidation is controlled correctly, the zirconium or titanium metal ions will form a zirconium or titanium transition metal oxide coating. This basic version of an new pretreatment will likely meet the needs of some finishing operations that use high-quality paint materials and finish a product where high corrosion resistance isn’t critical.
More demanding applications requiring substantial corrosion resistance, however, will require new pretreatments that also incorporate either additional transition metal oxides and/or adhesion promoters. Vanadium is an example of a transition metal used in new pretreatments that creates additional metal oxides to form on the substrate.
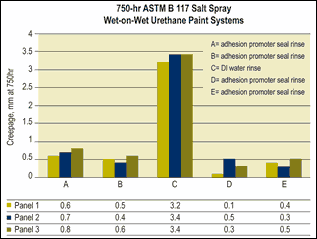
Adhesion promoters can be incorporated in new pretreatments or final seal rinse treatments. These materials, including silanes, polyacrylic acids and phosphonate, help to create an ortho-metallic bond between the substrate and paint film.
New pretreatments or final seal rinse treatments that contain adhesion promoters may provide enhanced performance under certain liquid paint systems compared with processes without the adhesion promoters. The difficulty with pretreatment and final seal rinse chemistries that contain adhesion promoters is ensuring compatibility of the resin used in the paint system and the adhesion promoter. If a finish line uses a variety of paint formulations with different types of resins, it can be difficult to produce equivalent results on all systems.
Another consideration when using adhesion promoters is that some studies have shown that the applied treatment should be a monolayer rather than a thick film, as a monolayer typically produces higher salt spray performance results.
Adhesion promoters can also create adhesion problems in finishing facilities that run previously painted rework back through the pretreatment system. In this case, the monolayer created on a rework part can act as a soil, thus creating adhesion problems.
Finally, if a major consideration in evaluating a new pretreatment is complete elimination of phosphate discharge, you will want to avoid chemistries that contain phosphonate components.
Process Considerations
In general, iron phosphate pretreatment formulations can be more forgiving than new pretreatments when there are production line stops. Frequent line stops will produce an uncontrollable condition for new pretreatments. The line stops will help to promote development of iron oxide coatings which, depending on the duration of the line stop, can result in flash or severe rusting of treated parts.
Again, the chemical reaction that occurs between the substrate and new pretreatments is a controlled oxidation process that’s completely different than a typical iron phosphate conversion process. Thus, one of the main benefits of new pretreatments is that you greatly reduce sludge build-up in the process bath, because less iron is dissolved from the ferrous substrate. This reduces labor costs associated with plugged nozzles and descaling of process equipment.
However, because new pretreatments dissolve less iron than traditional pretreatments, less surface area is created on the ferrous substrate for the paint to bind with. Corrosion testing of your particular paint system over a new pretreatment will determine if this presents an issue.
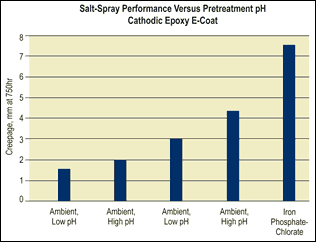
The main variable you should study as part of your testing matrix is the pH range of the pretreatment. In general, a lower pH range of 2.8–4.0 will produce superior corrosion resistance, in part because more “etching” occurs, creating more surface area. At this pH range, part appearance will be amber to yellow in color.
If you desire a pretreatment that produces a color similar to iron phosphate, a pH range of 4.0–6.0 will be necessary. Coatings that are produced in this higher pH range are light blue to dark blue in color, similar to a molybdenum-accelerated iron phosphate coating. However, the tradeoff may be diminished corrosion resistance.
New pretreatments operated in the higher pH range (4.0–6.0) can be used in mild steel process equipment. If your process requires the lower pH range for optimum corrosion performance, processing equipment will need to be constructed of stainless steel. You will need to discuss this with your chemical vendor to avoid potential equipment damage. Temperature can also work against you with new pretreatments. Field experience has shown that the temperature of the cleaner can create uncontrollable conditions.
Many existing metal pretreatment systems in the marketplace today were designed around the application of traditional iron or zinc phosphate technology. For example, the iron phosphate pretreatment step is typically used in stage three of a common five-stage spray washer. Many metal finishers have replaced the traditional iron phosphate treatment in stage three with a new pretreatment. Depending on the unique variables of each process, this may or may not work.
A five-stage process that runs the first-stage cleaning bath temperatures higher than 140°F may create considerable heat transfer. The heat is transferred into the following rinse stage by drag-out of chemical solution and by the parts themselves, causing the temperature of the rinse bath to rise. Parts being treated remain at high temperature because the rinse provides little cooling effect. Extremely hot parts that enter into the new pretreatment stage will cause the pretreatment to become overly aggressive. The end result can be rust formation instead of the desired transition metal oxide coating.
Having learned this in some cases the hard way, finishers who run high-temperature cleaning stages are placing the new pretreatment further down the process to allow the parts more time to cool before entering the new pretreatment bath. Increasing the rate of overflow on the rinse stage can also help in reducing heat transfer. However, raising water overflow of the rinse will increase operating costs and should be considered as part of any cost/benefit analysis.
Placing the new pretreatment further down the process will also help in minimizing pretreatment bath contamination. Large quantities of alkaline cleaner contamination will neutralize the acid levels in the new pretreatment bath. When this occurs and pH rises above 6.0, the zirconium and/or titanium will precipitate from the bath. This will dramatically lower corrosion resistance benefits.
Over time, iron will begin to build up in new pretreatment baths. It will eventually become too much for the chemistry to tolerate, and iron may redeposit back onto the substrate. A general guideline would be to have a maximum iron buildup of around 400 ppm. However, you should consult with your supplier, because the pretreatments may vary.
Note that if you decide to operate a new pretreatment in mild steel equipment, iron will also be dissolved from the equipment. This will add to the total contamination.
Substrate materials
Although this article has focused so far on ferrous metal substrates, new pretreatments are designed for use on nonferrous materials as well.
When controlled correctly, new pretreatments can and will produce quality results on cold-rolled steel. Pickled and oiled hot-rolled steel can also be successfully treated with new pretreatments; hot-rolled steel that has not been pickled and oiled will present problems. This type of hot-rolled steel typically has scale and smut that would be removed by an acid pickle process. The new pretreatments in general require a higher degree of part cleanliness prior to pretreatment than traditional iron or zinc phosphate conversion coatings. The presence of inorganic soils such as scale or smut on the substrate will prevent the new pretreatment from imparting a sufficient and uniform coating. This will lead to poor corrosion resistance and poor adhesion.
Fluorozirconic acid and fluorotitanic acid have been used for decades as treatments for nonferrous metal substrates such as aluminum and galvanized steel. Fluorozirconic acid and fluorotitanic treatment provide better corrosion results on these nonferrous substrates than traditional iron phosphating. The fluoride part of these acids is more effective at removing oxides found on aluminum and galvanized substrates than iron phosphate treatments. It has been common practice for years to add fluoride-based additives to iron phosphate treatments to improve adhesion on nonferrous substrates.
Finishers who coat primarily nonferrous substrate materials will want to closely monitor the fluoride level in the operating bath, as it will be consumed at a faster rate than the zirconium or titanium portion of the chemistry. You should discuss the amount of fluoride to be maintained in the new pretreatment bath with your chemical supplier, and then use a fluoride ion-specific probe to test this on a daily basis.
The Future
Ambient-temperature metal pretreatments have established a significant presence in today’s marketplace. Chemical suppliers will continue developing these technologies, making them acceptable for more paint systems.
There are also significant numbers of metal finishers who continue to operate traditional iron phosphate treatments designed to run at ambient temperature. Ambient-temperature zinc phosphate conversion coatings also have been recently introduced into the marketplace. Either of these offer a viable alternative for energy savings if phosphate discharge is not a concern.
All of these approaches should be considered when selecting the correct pretreatment system for your application. A full understanding of your options and the considerations discussed above will lead you to the best approach for your situation.
Read Next
Episode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More














.jpg;maxWidth=300;quality=90)











