Historical Highlights of Electroless Plating
In a 1984 article in Plating and Surface Finishing, for the AESF 75th anniversary year, Charles R. Shipley Jr. recounts the history of the commercialization of electroless plating beyond the discovery of electroless nickel by Dr. Abner Brenner and Grace Riddell at the National Bureau of Standards.
by
Charles R. Shipley, Jr.
Originally published as C.R. Shipley, Jr., Plating and Surface Finishing, 71 (6), 24-27 (1984).
Editor's Note: Here, Charles R. Shipley Jr. recounts the history of the commercialization of electroless plating beyond the discovery of electroless nickel by Dr. Abner Brenner and Grace Riddell at the National Bureau of Standards (published with this paper via http://short.pfonline.com/NASF18Jun2. A printable version of this paper can be accessed HERE.
The story of electroless plating begins in 1946 at the 34th Annual AES Meeting, when Abner Brenner and Grace Riddell of the National Bureau of Standards disclose results of their studies on experimental nickel electroplating baths.1 They had attempted to prevent undesirable oxidation of bath constituents at the inert anode by making additions of reducing agents to the bath. As luck would have it, one of the reducing agents explored was sodium hypophosphite. Surprisingly, the amount of nickel deposited exceeded the amount theoretically limited by Faraday's law. The rest is history!
They soon ascertained that nickel deposition occurred even when no external current was applied. It was evident that metal deposition was achieved by chemical reduction but, uniquely, the reduction process was dependent upon a catalytic surface. Once initiated, the nickel deposit was itself catalytic for continued reduction. The process was thus described as autocatalytic chemical reduction of metal ions to form a metal deposit. At first Brenner called the process "Electrodeless."
A year later at the 1947 Annual AES Meeting, the discoverers reported remarkable progress in the development of the new process.2 Bath compositions for autocatalytic deposition of nickel and cobalt were described, as were the effects of constituent concentrations, operating parameters and a number of other influential factors. This work led to two patents.3
During the presentation, it was suggested that the process be designated "Electroless," which is the term that has survived. In 1954, Brenner4 summarized the state of the art in his paper "Electroless Plating Comes of Age."
This dramatic discovery initiated the basis for a new industry - electroless plating - which today has become commercially important for finishing steel, aluminum, copper, plastics and many other materials. The use of electroless plating is continuing to grow, especially for electronic applications. Electroless plating complements electrolytic plating - sometimes used with it, sometimes competing with it. Major reasons for utilizing electroless in preference to electrolytic plating include:
- Uniform deposits over irregular surfaces.
- Direct plating on nonconductors.
- Deposition on isolated metal areas.
- Less porous, more corrosion-resistant deposits.
- Unique deposit properties.
- Bulk plating (barrels/baskets) and "semi-bulk" racking.
Today the most commercially important electroless deposits are nickel-phosphorus and copper. Nickel-boron, 99.7 percent nickel, cobalt and certain nickel-containing polyalloys also find considerable commercial use. Electroless plating is generally used for functional applications such as those illustrated in Fig. 1. Thin deposits of electroless metal are also used to provide a conductive base on molded plastic parts for decorative finishing.

Figure 1 - Electroless nickel-phosphorus is commonly used for functional applications such as these.
Production output from electroless plating often substantially exceeds that of electroplating because parts can be racked close together - up to as high as one square foot of work surface area per gallon of plating solution. Equipment and racking costs are much lower than for electroplating, and usually less floorspace is needed. Conversely, chemical costs are significantly higher because deposition is accomplished by chemical, rather than electrolytic, means. Also waste treatment is more involved.
Electroless nickel-phosphorus
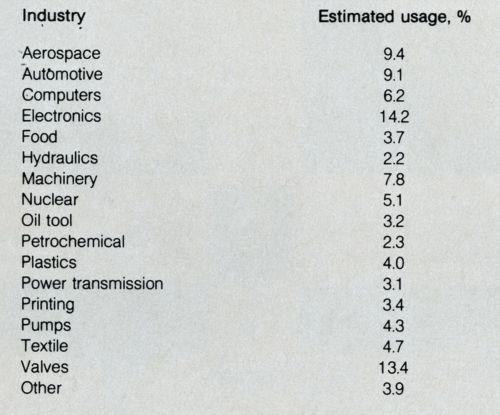
Electroless nickel-phosphorus (Ni-P) plating has emerged from little more than a laboratory curiosity in the early 1950s to a process utilized today in about 1000 installations in the U.S. alone. The largest electroless plating facility is probably in France, where a 100,000-gal tank is used to coat 20-ft-long tube bundles. Plating is performed for many industries (Table 1).5 It is estimated that over 70 percent of all electroless Ni-P plating is applied on mild steel, alloy steels and cast iron; 20 percent on aluminum and other non-ferrous metals; 6 percent on tool steel and stainless steel.
Table 1. Nickel-phosphorus plating usage (1984).
Chemistry
Gregoire Gutzeit and other associates at General American Transportation Co. performed considerable research on electroless Ni-P compositions and processes. A large number of patents were issued to them during the 1950s and 60s. The Gutzeit team found that a number of reagents have the faculty of considerably increasing deposition rates and so were called "exaltants";6 some of these agents also provided beneficial complexing and buffering action. Gutzeit and Krieg7 showed that, for optimum deposition rate, the concentration and ratio of hypophosphite and nickel salts were important. A major advance was the discovery8 by Talmey and Gutzeit that certain catalytic poisons, in trace concentrations, stabilize electroless nickel baths against the perplexing propensity to suddenly undergo spontaneous decomposition with the formation of black powder, resulting in a useless solution. In some instances, it was found that stabilizers might also increase the deposition rate and enhance deposit brightness.9 These studies led to the development of bath composition and processes for plating electroless Ni-P alloy under the tradename "Kanigen."
In 1959, Gutzeit9 reviewed the chemistry involved, and contributed a great deal of useful information. Other valuable summaries of electroless Ni-P deposition were published by ASTM10 in 1959, Saubestre11 in 1962, Gorbunova-Nikiforova12 in 1963, Pearlstein13 in 1974 and Gawrilov14 in 1979.
There are presently available many proprietary baths/processes for providing electroless Ni-P deposits of various characteristics. In general, the baths in common use are of the acid type, containing chelates, exaltants, buffers, stabilizers and other agents in addition to nickel salts and sodium hypophosphite. Operation is usually at 85 to 98°C (185 to 210°F) with deposition rates ranging from 10 to 20 μm/hr (0.4 to 0.8 mil/hr). Deposition is essentially linear with time. Replenishable, long-life proprietary baths were introduced during the late 1960s. Many of these proprietary baths are sophisticated formulations that provide a consistently fast plating rate, long-bath life for economy of operation, and the capability for producing deposits of high quality and reproducibility.
Deposit characteristics of Ni-P
Electroless Ni-P is generally known as "electroless nickel." This is a misnomer that undoubtedly has curtailed the use of this unique alloy. Nickel and Ni-P alloys are entirely different metals,15 as highlighted in Table 2. Their properties are significantly different, with Ni-P alloys possessing many unique properties and advantages. The differences are primarily due to the codeposited phosphorus content in the electroless alloys that ranges from about 3 to 12 percent, depending upon bath composition and operating parameters. The phosphorus content of deposits is increased by lowering bath pH9,16,17 and by increasing hypophosphite and orthophosphite concentrations. The most commonly used acid-type baths produce deposits containing 7 to 11 percent phosphorus; the lesser used alkaline-type baths produce deposits of about 3 to 7 percent. Bath temperature can also influence phosphorus content.17
Table 2 - Comparison of electroless versus electrolytic nickel deposits.

Deposits are often called amorphous because x-ray diffraction studies indicate an absence of a crystalline structure. Graham, Lindsey and Read18 systematically studied the structure and mechanical properties of electroless Ni-P deposits and concluded that deposits can be described as supersaturated solid solutions of phosphorus in a microcrystalline nickel matrix. Abrupt changes in mechanical properties such as tensile strength and ductility are observed at about a 7 percent phosphorus content. The hardness of electroless Ni-P deposits is about 500 to 600 kg/mm2, which is substantially harder than conventionally electrodeposited nickel. By heat treating for 1 hr at 400°C (750°F), hardness can be increased to more than 1000 kg/mm2. Increased hardness can also be obtained by heating at lower temperatures for a longer time, e.g., 16 hr at 280°C. Randin and Hintermann19 found that the wear resistance of electroless Ni-P coatings containing more than 7 percent phosphorus was excellent when heat treated at 400 to 600°C and was further improved with higher phosphorus content. These deposits have found use as an alternative to electrodeposited "hard" chromium for some wear-resistance applications. Electroless Ni-P deposits are not very ductile as plated, and ductility is further decreased when deposits are heat treated to greater hardness. Parker and Shah17 studied residual stress in electroless Ni-P deposits on various substrates as a function of phosphorus content, reporting that relatively low-stress deposits can be obtained as a function of bath composition and substrate.
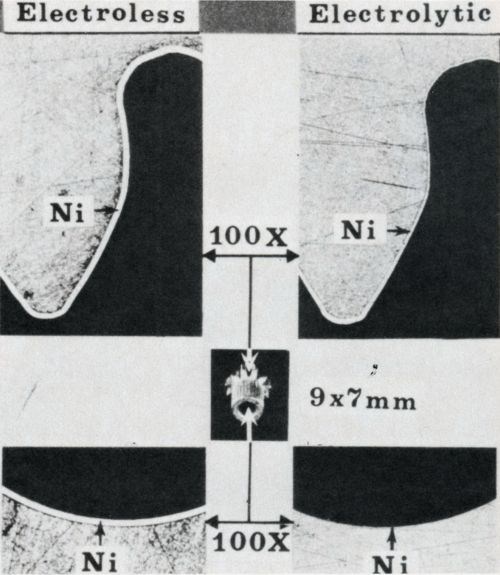
Figure 2 - Deposit uniformity of electroless Ni-P on gear teeth and inside hole vs. unsatisfactory throwing power of electrolytic nickel. Specimen shown in center is about 9 mm long and 7 mm in diameter across the teeth (see Ref. 13).
Deposit uniformity is a unique characteristic - indeed a hallmark - of all electroless plating processes. Many applications are related to this capability, as illustrated in Fig. 2. Unlike electroplating, uniform deposition can be obtained on irregular surfaces such as threads and on complex-shaped parts. Uniform deposits on areas such as tubing interiors, small holes and crevices are virtually impossible to achieve by electrodeposition, even when utilizing costly, complex auxiliary anode arrangements and high-current-density "thieving" devices.
Chemical resistance of electroless Ni-P is generally at least comparable to electrodeposited nickel. However, when containing 10 to 12 percent phosphorus, Ni-P deposits can provide superb corrosion protection for steels and other corrodible metals. Bath composition also affects resultant chemical resistance and corrosion prevention properties. Furthermore, electroless Ni-P deposits usually are less porous than electrodeposited nickel, and thus a given thickness will provide greater protection to corrodible substrates.20 Electroless Ni-P deposits have found numerous corrosion prevention applications for equipment used in the food processing, automotive, aircraft and petroleum industries.21,22 It was shown by Duncan23 that electroless Ni-P deposits containing 10 to 11 percent phosphorus are useful for protecting steel in the highly corrosive and aggressive oil-drilling environments. DiBari24 discussed automotive applications of electroless Ni-P alloys for providing wear resistance or solderability in addition to corrosion resistance. Double-layer electroless deposits on steel have been shown by Gruss and Pearlstein25 to offer improved corrosion resistance in marine environments.
An excellent compilation of data and discussion concerning the structure, physical properties, mechanical properties, fatigue strength and corrosion resistance was published by The International Nickel Company.26 Another compilation (Table 2) by Parker15 compares the properties of electroless Ni-P alloy and electrodeposited nickel. When considering the properties of electroless Ni-P deposits, it is important to keep in mind that Table 2 lists typical properties for alloys containing approximately 8 percent phosphorus. For example, the properties of an Ni-P alloy containing approximately 11 percent phosphorus are significantly different in several respects, including: increased corrosion resistance, substantially more non-magnetic, and somewhat higher electrical resistivity.
Deposition mechanism of Ni-P
There have been several mechanisms proposed to explain the electroless nickel deposition process. It has been well established that the amount of electroless Ni-P alloy deposited may approach, but is always less than, the molar equivalent of hydrogen gas evolved during deposition. This observation and the results of deuterium tracer studies27 explained the source of hydrogen evolved with regard to the reactants. From this data Lukes28 proposed a hydride transfer theory represented by the following equation:
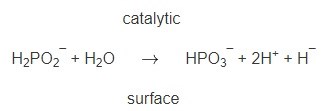
The hydride ion (H¯), on the catalytic surface, can react with available nickel ions by electron transfer to produce a nickel deposit and hydrogen gas evolution:
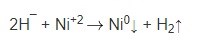
The overall reaction can thus be represented by:
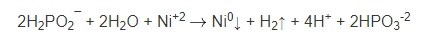
However, hydrogen ions can compete with nickel ions for reaction with hydride ion:
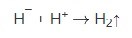
Therefore 2 gram-moles of hypophosphite can theoretically produce a maximum of 1-gram atomic weight of nickel; in actual practice, 2 gram-moles of hypophosphite can be expected to deposit about 0.7-gram atomic weight of Ni-P.
Electroless copper
The story of electroless copper - and also the story of plating on non-conductive substrates - began in 1947 when Harold Narcus29 wrote about "Practical Copper Reduction on Nonconductors." But it was not until the mid-1950s that electroless copper plating attained commercial acceptance when early formulations and processes were developed expressly for the manufacture of plated-through-hole (PTH) printed circuit boards. At that time the manufacture of printed circuits was a newly created, small, and struggling industry.
In 1955, electroless copper was being used for PTH circuits by an East Coast printed circuit shop, utilizing a home-brew formulation that Narcus was instrumental in developing. In 1956, the first commercially available proprietary electroless copper process, known as "Kopper Kold," was introduced on the West Coast. In the Midwest, Motorola was using a homebrew spray electroless copper for making semi-additive PTH circuit boards.
At the 1957 AES Annual Convention, Cahill30 described his success in producing autocatalytic chemical reduction of copper from alkaline copper tartrate baths using formaldehyde as the reducing agent. By coincidence, Abner Brenner was chairman of the session during which the paper was presented, and he remarked that the "process ought to have considerable use." How right he was!
By 1959, there was already great interest in electroless copper deposition for PTH interconnects of double-sided circuit boards as a replacement for mechanically inserted eyelets. At the 1959 AES Golden Jubilee Convention, Saubestre31 described a number of interesting experiments with electroless copper baths. However, the baths at that time had very limited life as cuprous oxide particles tended to form spontaneously in the bath, subsequently acting as catalytic nuclei for the formation of precipitous copper powder and resulting in rapid bath decomposition. It was clear that successful stabilization of the chemistry was a prerequisite for expanding the commercial use of electroless copper.
In the late 1960s, an important advance came with the development of fast-plating "heavy" electroless copper. This advancement eliminated flash copper electroplating, with its attendant time-consuming, re-racking and equipment-dependent requirements. Furthermore, the heavier thickness of electroless copper - typically 2 nm (about 80 microinches) - improved the reliability of void-free plated holes.
In 1967, a patent32 was issued to Schneble, Zeblisky, McCormack and Williamson for "ductile electroless copper" deposits having sufficient ductility to withstand at least 1.5 bends. This invention led to a new use for electroless copper - the manufacture of low-cost, fully additive printed circuits.
In the early 1970s, fast-plating electroless copper baths had progressed to the point where proprietary formulations were extremely stable, permitting "steady-state" operation. By 1980, automated, analytically based replenishment controllers were in use on many large-volume production lines.
Bath stabilization
In 1960, Agens33 reported that bubbling air stabilized electroless copper baths, presumably by oxidizing cuprous oxide particles, thereby preventing the formation of destabilizing catalytic nuclei. Bath aeration for this purpose has been used quite extensively. In the 1950s and 60s, it was found that mercaptobenzothiazole,34 thiourea,35 cyanide,36 divalent sulfur compounds37 and some other chemical agents were effective for stabilizing electroless plating baths. Stabilizers presumably function by complexing cuprous ions, or by catalytic deactivation of catalytic particles. In 1972, Saubestre38 reviewed and classified the various methods used to stabilize electroless copper baths.
Chemistry of electroless copper
Electroless copper baths in commercial use are formaldehyde based. Since the required reduction potential of formaldehyde increases with alkalinity, baths are usually operated at a pH above 12. Because of this it is necessary to use complexing agents (chelates) to prevent cupric hydroxide precipitation. Complexing agents affect bath stability, deposition rates, quality of copper deposits, and so are very important. In addition to tartrates, the most commonly used complexing agents include EDTA, Quadrol and alkanolamines for which patents were issued to Atkinson39 in 1964, Lukes40 in 1961 and Dutkewych41 in 1968.
Most commercially available electroless copper baths tend to plate quite brittle deposits, especially noticeable when relatively thick. Some additives, most notably sodium cyanide, have been found capable of improving deposit bendability. In 1971, Grunwald, Rhodenizer and Slominski42 investigated physical properties of electroless copper and concluded that, in a given bath, an increase in plating temperature or a decrease in plating rate can increase the ductility of the deposits (as measured by bending). At elevated bath temperatures the volatility of formaldehyde becomes particularly troublesome; in 1973, the use of a formaldehyde addition agent was patented to Shipley, et al.43 for the purpose of minimizing formaldehyde losses and suppressing fumes.
Surface active agents are often included in bath formulations to help improve deposit qualities, having been suggested by Lukes40 in 1961.
In 1959, Saubestre31 investigated a number of reducing agents for electroless copper deposition, and concluded that further study was warranted with hydrazine, hypophosphite and hyposulfite. DMAB is another reducing agent that might warrant further study.
Deposition mechanism of electroless copper
For each gram atomic weight of copper plated electrolessly at least 2 moles of formaldehyde and 4 moles of hydroxide are consumed, while at least 1 mole of hydrogen is evolved:

In actual practice, there will be more formaldehyde and hydroxide consumed because of disproportionation into methanol and formate (Cannizzaro reaction) and because of volatilization losses and scavenging of carbon dioxide. The actual mechanism of deposition may involve transfer of hydride44 from formaldehyde at the catalytic surface, similar to the mechanism postulated for electroless nickel-phosphorus.
Applications
Electroless copper is most often deposited on non-conductors as a conductive base for subsequent electrolytic plating. The use of thin electroless copper has continued to increase during the last 25 years. It is estimated that over 300 million square feet of surface area is now being plated annually with thin electroless copper.

Figure 3 - Grille shows beauty and practicality achieved by plating on molded plastic. Elec-troless copper is used as conductive base because it permits long life in corrosive environments.
Electroless copper is preferred over electroless nickel as the initial conductive layer for decorative electroplated plastic products subjected to prolonged corrosive environmental exposure (e.g., the auto grille shown in Fig. 3). At the 1969 ASEP annual meeting, Coombes45 presented corrosion test data that showed electroless copper provides vastly superior life for plated plastic parts. Corroborative results were later reported by Wedel,46,47 who critically reviewed the processes involved in producing electroplated plastics and discussed the factors influencing adhesion and durability. The Coombes and Wedel findings are credited for the acceptance of plated plastic exterior parts by the automotive industry.
Thin electroless copper is now finding use in the functional plating of plastics, such as computer packages and other electronic devices, for the purpose of controlling electromagnetic interference (EMI). The use of electroless copper for this purpose was suggested by Lordi48 in 1966, and further studied by Hajdu and Krulik.49 EMI shielding applications differ from decorative plated plastics in that the thin electroless copper deposit (typically about 1 nm thick) serves as the effective conductive layer. To prevent expected oxidation the electroless copper deposit is coated with a protective plastic coating and/or by a thin protective metal coating that can be applied by electrolytic, electroless or immersion plating as known in the art.
Thick electroless copper has been used for many years for making fully additive printed circuit boards. Usage has been restricted to lower quality PC boards - evidently because of insufficient elongation in the electroless copper deposits, thereby resulting in microscopic fracturing or other defects within the hole barrels as a result of subsequent soldering. A more ductile electroless copper is needed for high-quality, fully additive PC boards that are capable of withstanding solder shock. This need is emphasized by the decreasing hole diameters as depicted in Fig. 4, and also by increasingly high-density circuitry (narrow lines and spacings).

Figure 4 - Trends in PTH aspect ratios.
Electroless cobalt
This history began in 1947, when Brenner and Riddell2 disclosed that cobalt-phosphorus deposits can be produced electrolessly from alkaline baths operated at pH 8 to 10. In 1962, Fisher and Chilton50 used electroless cobalt-phosphorus processes to produce high-coercive-force deposits useful for high-density recording. In 1964, Ranson and Zentner51 described deposits of low coercive force with potential application for computer storage elements of high-speed switching devices. In the mid-1960s, it was found that deposits with wide variations in magnetic properties could be obtained,52-56 resulting from changes in bath constituent concentration and the pH, or by judicious use of addition agents. In 1974, Pearlstein and Weightman57 developed a bath with dimethylamine borane as the reducing agent that, unlike the hypophosphite baths, was capable of depositing electroless cobalt-boron in acid solutions. Commercial usage of electroless cobalt evidently is limited to magnetic applications, especially to thin magnetic deposits on tape, discs and wire substrates. Cobalt-boron deposits on steel are considered promising for corrosion prevention.
Electroless nickel-boron
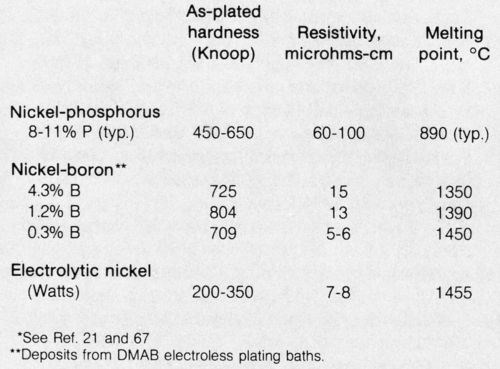
Electroless nickel-boron alloy plating dates back to 1958,58 but it was not until about 1968, when proprietary plating solutions became available, that commercial interest started in the U.S. A number of patents issued during the 1960s, including composition patents as well as a Berzins patent59 in 1962 for nickel-boron alloy plate containing about 1 to 8 percent boron. Deposits containing at least 3 percent boron are considerably harder in the as-plated condition21 (Table 3) and somewhat harder after heat treating than nickel-phosphorus deposits. Nickel-boron is used for some wear-resistance applications, including those on high-strength aluminum alloys where heat treatment is precluded.
Table 3 - Comparison of nickel deposits.*
Baths that utilize borohydride as the reducing agent are operated at pH 12 to 14 for the reason that borohydride decomposition by hydrolysis occurs at lower pH. Complexing agents60-62 are required to prevent nickel hydroxide precipitation. Alloy deposits from borohydride baths typically contain from 3 to 8 percent boron. In 1966, Lang63 described commercial applications for such deposits. However, interest in the borohydride process appears to have waned, and there is little reported activity in the U.S. at the present time.
Baths that utilize amine boranes as the reducing agent were developed64-66 in the 1960s. Dimethylamine borane (DMAB) baths are operated at pH 5 to 11 with temperatures below 75°C. At lower pH, or at higher temperature, losses of DMAB by hydrolysis may be significant. In 1971, Mallory67 thoroughly investigated DMAB baths for effects of composition and operating parameters.
DMAB baths deposit nickel-boron alloys containing from as little as 0.1 up to 5 percent boron, depending upon pH and/or bath composition. Deposits containing about 0.3 percent boron - i.e., 99.7 percent nickel by weight - exhibit properties that are quite identical to nickel metal. For example, the melting point of 99.7 percent nickel/0.3 percent boron plate is about the same as for pure nickel (1455°C) and electrical properties are virtually identical. Melting points have been reported to be 1350°C14 and 1080°C14 for nickel-boron alloy deposits containing 4.3 and 5 percent boron, respectively.
In spite of higher cost, amine borane baths have found an important niche. In 1983, Baudrand21 summarized prospective uses for electroless nickel-boron.
Electroless polyalloys
Alloy deposits offer the opportunity for achieving a wide variety of new and useful properties. In 1973, Gulla68 showed that an electroless nickel-copper-phosphorus alloy deposit containing only about 1 percent copper provided a number of unique properties. The deposits can be microsmooth, bright and somewhat more ductile, and can provide excellent corrosion resistance. This alloy, which has very low ferromagnetism, has found application as a base coating on polished aluminum for magnetic recording discs.
Nickel-cobalt-phosphorus alloy deposits may be produced from alkaline baths in all proportions of cobalt and nickel, since each metal is independently capable of electroless deposition. Cobalt is not deposited independently nor is it codeposited with nickel from acid-type hypophosphite baths. A number of other metals that cannot be independently deposited can be codeposited in alloys also containing nickel and phosphorus or boron. For example, Pearlstein and Weightman69 reported in 1965 that up to 20 percent tungsten, 46 percent rhenium, 15 percent zinc or 2 percent tin can be codeposited with electroless nickel-phosphorus. In the 1960s, it was reported70-74 that ternary alloys of electroless cobalt-phosphorus with nickel, tungsten, rhenium, iron or zinc provided deposits with interesting magnetic properties. In 1966, Schmeckenbecher75 reported that electroless nickel-iron alloys have magnetic properties useful for information storage. In the 1970s, Mallory76'77 introduced ternary and quaternary alloys of nickel with phosphorus, molybdenum, tungsten and tin in various combinations. Deposits of 74 percent Ni-16 percent P-8 percent Sn-2 percent Cu or 83 percent Ni-9 percent W-8 percent P on steel or aluminum were claimed to provide superior corrosion resistance.
In the 1970s, Mallory76-78 thoroughly investigated DMAB baths for the capability to produce potentially useful "polyalloys." Polyalloys of nickel, boron and molybdenum or tungsten are suitable for ultrasonic bonding and solderability. These deposits also have unique ductility and provide superior corrosion resistance. Nickel-tin-boron and nickel-tin-tungsten-boron alloys have easy solderability. Nickel-molybdenum-boron spontaneously deposits on clean copper without catalytic activation.
In 1981, Matsuoka and Hayashi79 reported that a nickel-boron-thallium alloy can be produced electrolessly from a bath utilizing pyridine borane as a reducing agent and thallous nitrate as a stabilizer.
In 1979, Baudrand80 summarized prospective uses for polyalloys.
Electroless composites
Composite coatings can be produced electrolessly by physically suspending finely divided particles in an electroless nickel-phosphorus bath during deposition. In the early 1970s, Metzger, Ott, Pappe and Schmidt patented81 a family of electroless composites with a great many different kinds of particulate materials. In 1974, Parker82 conducted tests on electroless nickel-phosphorus deposits containing occluded graphite, chromium carbide, tungsten carbide, aluminum oxide, titanium carbide, silicon carbide, boron carbide, diamond or Teflon. The carbides and diamond were generally superior in wear resistance to hard chromium, particularly when the deposit was heat treated to increase hardness. Most current interest appears to be in silicon carbide or diamond composites.83,84 Feldstein85 in 1983 reviewed the historical developments and state of the art of electroless composite deposits for wear-resistance applications.
Other electroless metals
Palladium, gold, silver, tin and pure nickel are other metals that deserve mention with regard to electroless plating.
• Electroless palladium: Baths for electroless deposition of palladium were reported by Rhoda in 1961, utilizing hydrazine,86 by Sergienko in 1968, utilizing hypophosphite87 and by Pearlstein and Weightman88 in 1969 and Hough89 in 1981, utilizing tertiary amine boranes as reducing agents. However, there is no reported commercial interest in these baths, even though the deposits would seem to have useful applications for electrical contacts, connectors and tab plating.
• Electroless Gold: Autocatalytic gold deposition was accomplished by Okinaka90 in 1970, by McCormack91 in 1971 and by El-Shazly and Baker92 in 1982, using amine borane or borohydride reducing agents. Reportedly there is some commercial application in the fabrication of semiconductor devices. In 1982, a paper by Molenaar93 described a wide range of electroless gold-copper alloys, containing up to 98 percent gold, using formaldehyde as the reducing agent.
• Electroless Silver: Electroless silver deposits were successfully produced in 1974 from a dimethylamine borane bath94 by Pearlstein and Weightman.
• Electroless Tin: In 1980, Warwick and Shirley95 reported on experiments for electroless tin plating using titanium trichloride as a reducing agent, and in 1981, a patent issued to Molenaar96 claiming autocatalytic deposition of tin.
• Electroless Nickel: In the early 1960s, Levy97 and Dini98 reported that pure nickel deposits can be produced electrolessly by utilizing hydrazine as the reducing agent. Unlike hypophosphite, borohydride, amine boranes and pyridine borane, the use of hydrazine as the reducing agent results in the electroless deposition of metal without being alloyed. Hydrazine compositions tend to be very unstable. No known commercial activity has been reported.
Plating on nonconductors
This important application of electroless plating is made possible by rendering the nonconductive surfaces catalytically active, so as to initiate electroless metal deposition. In 1955, Pearlstein99 described catalytic activation by a two-step process: immersion in stannous chloride solution, followed by immersion in palladium chloride solution after an intervening rinse. Subsequent immersion in a suitable electroless plating bath results in metal being deposited onto the catalyzed surface. In 1958, a colloidal tin-palladium one-step catalyst100 was commercially introduced. This process, which increased the efficiency and reliability of PTH manufacturing and plastic plating, is still the most widely used today.
In 1972, Groshart101 reviewed the history of metallizing nonconductors and the preparation of plastics for plating. There have been many commercially important developments in processes for plastic plating. Particularly noteworthy are those for decorative plating on ABS plastic, including the development of plating-grade ABS in the mid-1960s, the use of chromic acid for etching ABS, and the earlier advent of ductile, leveling electrolytic copper plating baths. Also, processes have been developed for plating on several other moldable plastics, including Noryl, nylons, polyester, polycarbonate and polysulfone. Similarly, technological advances in PTH processes (new conditioners/etchback treatments) are now being used in the manufacture of highly complex, multilayer circuit boards.
Electroless copper is the universal choice for printed circuit applications. For decorative plating on plastics, both electroless nickel-phosphorus and electroless copper are widely used, depending upon the application. Electroless copper is being used for EMI shielding applications, often in combination with electroless nickel-phosphorus.48
The future of electroless plating
Commercial usage of electroless plating will grow quite rapidly during the next 10 years. Applications will increase in number and size, especially as design engineers and advanced engineering groups learn more about the capabilities and advantages of electrolessly plated metals and alloys. Technological advances will continue for electroless plating and will further increase usage. Here are a few predictions:
- New and better electroless plating solutions will be formulated to meet technological requirements.
- Functional plating of engineering-grade plastics will become increasingly important (e.g., for EMI shielding).
- More "platable" plastics will become available.
- There will be new electroless processes for PTH circuits, including high-quality additive circuit processes.
- Users will specify electroless plating more and more for high-performance applications, high-technology uses and high corrosion resistance.
- Plating of electroless nickel alloys on metal substrates will increase substantially, especially on aluminum.
- Electroless nickel-phosphorus will replace cadmium plating for many applications.
The "future history" of electroless plating promises to be very exciting!
References
1. A. Brenner and G.E. Riddell, Proc. AES, 33, 23 (1946).
2. A. Brenner and G.E. Riddell, Proc. AES, 34, 156 (1947).
3. A. Brenner and G.E. Riddell, U.S. patents 2,532,283-4 (1950).
4. A. Brenner, Met. Finish., 52, 68 (Nov. 1954); 52, 61 (Dec. 1954).
5. D.A. Ehrhardt, Proc. Electroless Nickel Conference (Nov. 1979).
6. G. Gutzeit and E.J. Ramirez, U.S. patent 2,658,842 (1953).
7. G. Gutzeit and A. Krieg, U.S. patent 2,658,841 (1953).
8. P. Talmey and G. Gutzeit, U.S. patent 2,762,723 (1956).
9. G. Gutzeit, Plating, 46, 1158, 1275, 1377 (1959); 47, 63 (1960).
10. "Symposium on Electroless Nickel Plating," ASTM Spec. Tech. Publ. No. 265, ASTM, West Conshohocken, PA (1959).
11. E.B. Saubestre, Met. Finish., 60, 67 (June 1962); 60, 49 (July 1962); 60, 45 (Aug. 1962); 60, 59 (Sept. 1962).
12. K.M. Gorbunova and A.A. Nikiforova, Physiochemical Principles of Nickel Plating, Israel Program for Scientific Translations, Jerusalem, 1963; TT 63-1-1003.
13. F. Pearlstein, "Electroless Plating," in (F.A. Lowenheim, ed.) Modern Electroplating, 3rd ed., John Wiley & Sons, New York, NY, 1974; chap. 31.
14. G.G. Gawrilov, Chemical (Electroless) Nickel Plating, Portcullis Press Ltd., Surrey, UK, 1979.
15. K. Parker, Proc. Electroless Nickel Conference (Nov. 1979).
16. C. Baldwin and T.E. Such, Trans. Inst. Met. Finish., 46, pt. 2, 73 (1968).
17. K. Parker and H. Shah, Plating, 58, 230 (1971).
18. A.H. Graham, R.W. Lindsay and H.J. Read, J. Electrochem. Soc., 112, 401 (1965).
19. J.P. Randin and H.E. Hintermann, Plating, 54, 523 (1967).
20. C.H. de Minjer and A. Brenner, Plating, 44, 1297 (1957).
21. D.W. Baudrand, Plat. Surf. Finish., 70, 24 (Dec. 1983).
22. R.N. Duncan, Products Finish., 46, 46 (Sept. 1982).
23. R.N. Duncan, Mater. Perform., 22, 28 (Jan. 1983).
24. G.A. DiBari, Met. Finish., 81, 31 (Dec. 1983).
25. L. Gruss and F. Pearlstein, Plat. Surf. Finish., 70, 47 (Feb. 1983).
26. Technical Bulletin, "The Engineering Properties of Nickel," The International Nickel Co., New York, NY 10004 (1971).
27. A.A. Sutyagina, K.M. Gorbunova and M.P. Glazunov, Russ. J. Phys. Chem., 37, 1096, 1196 (1963).
28. R.M. Lukes, Plating, 51, 969 (1964).
29. H. Narcus, Met. Finish., 45, 64 (Sept. 1947).
30. A.E. Cahill, Proc. AES, 44,130 (1957).
31 E.B. Saubestre, Proc. AES, 46, 264 (1959).
32. F.W. Schneble, R.J. Zeblisky, J.F. McCornnack and J.D. Williamson, U.S. patent 3,310,430 (1967).
33. M.C. Agens, U.S. patent 2,938,805 (1960).
34. F. Pearlstein, U.S. patent 3,222,195 (1965).
35. M. Saito, Prod. Finish., 31, 7, 57 (1967).
36. R.J. Zeblisky, J.F. McCormack, J.D. Williamson and F.W. Schneble, U.S. patent 3,095,309 (1963).
37. F.W. Schneble, R.J. Zeblisky, J.F. McCormack and J.D. Williamson, U.S. patent 3,361,580 (1968).
38. E.B. Saubestre, Plating, 59, 563 (1972).
39. R.B. Atkinson, U.S. patent 3,119,709 (1964).
40. R.M. Lukes, U.S. patent 2,996,408 (1961).
41. O.B. Dutkewych, U.S. patent 3,383,224 (1968).
42. J.J. Grunwald, H. Rhodenizer and L. Slominski, Plating, 58, 1004 (1971).
43. C.R. Shipley Jr., L.H. Shipley, M. Gulla and O.B. Dutkewych, U.S. patent 3,728,137 (1973).
44. R.M. Lukes, Plating, 51, 1066 (1964).
45. R.L Coombes, Proc. ASEP 2nd Annual Mtg. (1969).
46. R.G. Wedel, Plating, 62, 40 (Jan. 1975); 62, 235 (March 1975).
47. R.G. Wedel, Int. Met. Rev., 22, 97 (June 1977).
48. G.A. Lordi, Plating, 54, 382 (1967).
49. J. Hajdu and G. Krulik, Plat. Surf. Finish., 70, 42 (July 1983).
50. R.D. Fisher and W.H. Chilton, J. Electrochem. Soc., 109, 485 (1962).
51. LID. Ransom and V. Zentner, J. Electrochem. Soc., 111, 1423 (1964).
52. Y. Moradzadeh, J. Electrochem. Soc, 112, 891 (1965).
53. M.G. Miksic, R. Travieso, A. Arcus and R.H. Wright, J. Electrochem. Soc., 113, 360 (1966).
54. J.S.,Sallo, Plating, 54, 257 (1967).
55. J.S. Judge, J.R. Morrison, D.E. Speliotis and J.R. Depew, Plating, 54, 533 (1967).
56. R.D. Fisher and W.H. Chilton, Plating, 54, 537 (1967).
57. F. Pearlstein and R.F. Weightman, J. Electrochem. Soc., 121, 1023 (1974).
58. Deutsches Auslegeschrift (DAS) 1,137,918 (1958).
59. T. Berzins, U.S. patent 3,096,182 (1963).
60. E.A. Sullivan, U.S. patent 2,942,990 (1960).
61. T. Berzins, U.S. patent 3,045,334 (1962).
62. H. Narcus, Plating, 54, 380 (1967).
63. K. Lang, Electroplat. Met. Finish., 19, 3, 86 (1966).
64. R.M. Hoke, U.S. patent 2,990,296 (1961).
65. W.J. Cooper, J.C. Renforth, R.J. Varga and A.E. Weber, Tech. Bull. CCC-AB-1, "Electroless Plating with Amine Boranes," Callery Chemical Co., Callery, PA 16024 (1968).
66. T. Berzins, U.S. patent 3,338,726 (1967).
67. G.O. Mallory, Plating, 58, 319 (1971).
68. M. Gulla, U.S. patents 3,764,352 (1973); 3,832,168 (1974).
69. F. Pearlstein and R.F. Weightman, Electrochem. Technol., 6, 427 (1965).
70. F. Pearlstein and R.F. Weightman, Plating, 54, 714 (1967).
71. J.R: DePew and D.E. Speliotis, Plating, 54, 705 (1967).
72. R.J. Heritage and M.T. Walker, J. Electron. Control, 7, 543 (1960).
73. J. Ragrowski and M. Lauriente, J. Electrochem. So.c, 109, 987 (1962).
74. G.W. Lawless and R.D. Fisher, Plating, 54, 709 (1967).
75. A.F. Schmeckenbecher, J. Electrochem. Soc., 113, 778 (1966).
76. G.O. Mallory Jr., U.S. patent 4,019,910 (1977).
77. G.O. Mallory Jr. and T.R. Horhn, Plat. Surf. Finish., 66, 40 (April 1979).
78. G.O. Mallory, Plat. Surf. Finish., 63, 34 (June 1976).
79. M. Matsuoka and T. Hayashi, Plat. Surf. Finish., 68, 66 (July 1981).
80. D.W. Baudrand, Plat. Surf. Finish., 66, 18 (Nov. 1979).
81. W. Metzger, R. Ott, G. Pappe and H. Schmidt, U.S. patents 3,617,363 (1971); 3,753,667 (1973).
82. K. Parker, Plating, 61, 834 (1974).
83. F.N. Hubbell, Plat. Surf. Finish., 65, 58 (Dec. 1976).
84. N. Feldstein, T. Lancsek, R. Barras, R. Spencer and N. Bailey, Prod. Finish., 44, 65 (July 1980).
85. N. Feldstein, Met. Finish., 81, 35 (Aug. 1983).
86. R.N. Rhoda, J. Electrochem. Soc., 108, 707 (1961).
87. A. Sergienko, U.S. patent 3,418,143 (1968).
88. F. Pearlstein and R.F. Weightman, Plating, 56, 158 (1969).
89. W.V. Hough, J.L. Little and K.E. Warheit, U.S. patent 4,255,194 (1981).
90. Y. Okinaka, Plating, 57, 914 (1970).
91. J.F. McCormack, U.S. patent 3,589,916 (1971).
92. M.F. El-Shazly and K.D. Baker, U.S. patent 4,337,091 (1982).
93. A. Molenaar, J. Electrochem. Soc., 129, 1917 (1982).
94. F. Pearlstein and R.F. Weightman, Plating, 61, 154 (1974).
95. M.E. Warwick and B.J. Shirley, Trans. Inst. Met. Finish., 58, 9 (1980).
96. A. Molenaar, U.S. patent 4,269,625 (1981).
97. D.J. Levy, Electrochem. Technol., 1, 38 (1963).
98. J.W. Dini and P.R. Coronado, Plating, 54, 385 (1967).
99. F. Pearlstein, Met. Finish., 53, 59 (Aug. 1955).
100. C.R. Shipley Jr., U.S. patent 3,011,920 (1961).
101. E. Groshart, Met. Finish., 70, 54 (Jan. 1972); 70, 85 (Feb. 1972); 70, 46 (March 1972); 71, 60 (Feb. 1973); 71, 51 (March 1973).
102. J.L. Adcock, AES Illustrated Lecture No. 44, "Electroplating Plastics" (1980).
About the author (written in 1984)

Charles R. Shipley Jr. is chairman of the board at Shipley Company, Inc., Newton, Massachusetts. He co-founded the company in 1957 and has contributed to many new product developments of commercial value in the fields of electroless plating and positive-working photoresist. He is listed as the sole or joint inventor on 19 U.S. patents. In recognition of his catalyst process for the plating-on-plastics industry, Mr. Shipley received the ASEP Award of Merit in 1974. Also, he was recently awarded an honorary Doctor of Science degree from Clarkson College, in Potsdam, New York.
Related Content
Innovation in Plating on Plastic
Plating on advanced plastics solution offers improved adhesion, temperature resistance and cost savings.
Read More3 Tests to Ensure Parts are Clean Prior to Plating
Making sure that all of the pre-processing fluids are removed prior to plating is not as simple as it seems. Rich Held of Haviland Products outlines three tests that can help verify that your parts are clean.
Read MoreLiquid Chrome Vs. Chromic Acid Flake
Contemplating how to continue offering chromic acid services in an increasingly stringent regulatory world? Liquid chrome products may be the solution you’re looking for.
Read MoreProducts Finishing Reveals 2023 Qualifying Top Shops
Each year PF conducts its Top Shops Benchmarking Survey, offering shops a tool to better understand their overall performance in the industry. The program also recognizes shops that meet a set of criteria to qualify as Top Shops.
Read MoreRead Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read More





















