Identifying New Alternatives for Chromium Plating
A comprehensive review of chromium plating alternatives and a new look at a long-forgotten but potentially important process - iron plating
by Per Møller, Technical University of Denmark, Lyngby, Denmark and Lars Pleth Nielsen, Danish Technological Institute, Aarhus, Denmark
Editor’s Note: This paper is a peer-reviewed and edited version of a presentation delivered at NASF SUR/FIN 2014 in Cleveland, Ohio on June 10, 2014. A printable PDF version is available by clicking HERE.
ABSTRACT
Many industries are looking for alternatives to hard chromium like thermochemical diffusion and PVD. However, it is hard to find one replacement coating fitting all applications where hard chromium is normally the preferred solution. This is simply because hard chromium is not only price competitive but also has several unique properties, such as good corrosion resistance, high hardness, high abrasive wear resistance and good low friction properties, which are all desirable at the same time. In this paper, we present a new iron-phosphorus alloy with 1-6 wt% P produced by an electroplating process, followed by electrodeposition of a topcoat of tin (Sn) of a few microns thickness, subsequently diffusion annealed at 220°C. Characterization of the new coating has revealed that some of the properties known from hard chromium such as low friction, high hardness, excellent resistance against adhesive wear, good ability to retain lubricants and high durability in neutral salt spray tests can actually be matched by this new FeP/Sn alloyed coating.
Keywords: hard chromium plating, substitutes, alternatives, REACH, iron plating, iron-phosphorus, tin, diffusion annealing
Introduction
Chromium is a hard metal, and is too brittle to be used as a construction material in its pure form. Nevertheless, it finds widespread application as an alloying element in steel and as a surface coating in the form of chromium plating. Stainless steel contains a significant amount of chromium (minimum 10-12 wt%). Additional elements such as nickel can be added to improve the mechanical properties, whereas, e.g., molybdenum can be added in order to improve the corrosion resistance.
Chromium plating can be used in decorative applications to obtain a highly reflective glossy surface (called bright chromium plating) or for engineering applications to obtain a hard, wear-resistant and corrosion resistant surface. The type of chromium plating applied critically depends on the functionality and application of the component in question. Hard chromium coatings have numerous applications and are used on rollers, hydraulic cylinders and various other industrial components and tools.
Hydraulic cylinders are commonly coated with 20-30 µm of hard chromium to counteract abrasive wear. During operation, dust will settle and accumulate on the chromium plated surface. The dust will later be trapped by the oil gasket when the piston moves in and out of the cylinder pipe. The type of wear can be classified as two- or three-part abrasive wear, depending on whether or not the abrasive particle is incorporated into the sealing gasket.
The main difference between decorative and hard chromium is the layer thickness, which is highly dependent on the specified function of the coating. Normally, bright chromium plating will vary from 0.25 to 1.0 μm, and typically hard chromium plating will typically vary between 20-30 µm, but can in special cases be as much as 1000 μm.
The sunset date for using chromium trioxide (Cr+6) in Europe is September 21, 2017. After this date, it will be no longer permissible to use hexavalent chromium for any production in the surface treatment field without having an authorization. March 21, 2016 is the latest date to apply for such an authorization. If an authorization is required, it is specific to a substance and its application(s). It is important to note that there are no application-specific exceptions from authorization granted. This implies that any use of chromium trioxide will be subject to authorization. This also means that each application of a substance requires its own authorization. In the case of chromium trioxide, decorative and functional chromium plating will be separate applications for each type of device, implying that each new product will require its own specific authorization. At present, it is not clear if further detailing into specific applications of hard chromium is required. For example, it may be necessary to specify the sector where hard chromium plating is used in segments, such as aerospace applications, hydraulic pistons, automotive engine components, etc.
Basically each chromium plater needs to be authorized to use chromium trioxide for his own applications. However, authorization for a particular use can be shared throughout the supply chain. For example, if the importer of chromium trioxide applies for, and is granted, authorization for the electroplating of hard chromium for a specific application, although this is not the importer’s own use, then each hard chromium plating shop using material from this importer is compliant with the pre-authorization requirements without having the necessary authorization itself.
As the process of authorization is very complex, costly and time consuming, the industry is advised to collaborate along the relevant supply chains. Close and well organized cooperation of all concerned users of chromium trioxide - importers, formulators and platers - is the key to achieving sound application documents and consistent information.
The reason that hexavalent chromium needs an authorization is the REACH Annex XIV list. REACH is the abbreviation for Registration, Evaluation, Authorization and restriction of CHemicals. REACH entered into force on June 1, 2007, with a phased implementation over the next decade. The regulation also established the European Chemicals Agency, which manages the technical, scientific and administrative aspects of REACH (Fig. 1).

Figure 1 - Background information from the REACH directive.
REACH addresses the production and use of chemical substances and their potential impact on both human health and the environment. Its 849 pages took seven years to consolidate, and it has been described as the most complex legislation in the European Union's history and the most important one for the last 20 years. It is the strictest law to date regulating chemical substances and will affect industries throughout the world.
REACH also addresses the continued use of chemical Substances of Very High Concern (SVHC) because of their potential negative impacts on human health or the environment. This type of chemicals is added annually to the so-called REACH Annex XIV list. Some uses of SVHCs may be subject to prior authorization from the European Chemicals Agency, and applicants for authorization will have to include plans to replace use of the SVHC chemicals with safer alternatives (or, if no safer alternative exists, the applicant must work to find one), known as substitution. Hexavalent chromium became part of this list in 2011. The motivation for including hexavalent chromium in the REACH Annex XIV list is discussed further below.
Trying to read and understand the background for this decision will soon make it clear that surface technologies based on hexavalent chromium will not be part of the future. From a safety point of view it makes sense to remove dangerous chemicals from consumers. In particular, chromated zinc, aluminum and magnesium are significant. However, removing hard chromium from technical application will introduce numerous material science problems and issues and it will in many cases be hard to find functional alternatives.
The history of hard chromium plating
Since Colin G. Fink received U.S. Patent 1,581,188 in 1926, many applications using hard chromium coatings have been developed and introduced. Many of them have been identified by trial and error, without a deeper understanding of the underlying materials properties. One of the first patented applications, U.S. Patent 1,746,751A, titled. "Film-forming Element," was originally assigned to Eastman Kodak Co. with Henry E. Van Derhoef as the inventor. The idea behind the patent was to produce an easy release coating for cellulose nitrate, which was the material used for silent movie production in the beginning of the film era. The citation from the patent reads:
“I discovered that a very smooth chromium surface (which is, of course, quite hard) is very suitable for the production of films by deposition of a dope thereon containing the cellulose derivative, such as cellulose nitrate, acetate or ether.
“With such a chromium surface I found that the film need not be completely freed of solvents before removal (as in the case of a hard smooth glass surface) but that the film could be stripped from the chromium surface while the film was quite “green,” i.e., while the film still contained a good portion of its solvents although having sufficient body to be handled. This also was not to be predicted by past experience with nickel or silver surfaces as such metals are quite soft when compared with chromium or glass.”
This patent is the first literature stating that a chromium coating has easy release properties, which is now understood and characterized by a surface property called surface energy. What Eastman Kodak Co. is stating is the fact that a chromium coating has a "low surface energy" (Fig. 2). These properties have made hard chromium very attractive for printing rollers and in tools, where easy release properties are important.

Figure 2 - The water drop (top) has a contact angle of less than 90°, indicating a substrate material with a "higher surface energy." The droplet (below) has a contact angle greater than 90°, indicating that the substrate material has a "low surface energy."
The properties of hard chromium
If one should try to describe the main properties, which, over nearly 90 years, have made hard chromium very popular, they are (1) its high hardness as compared to many other coatings, (2) the ability to apply the coating in large thicknesses of over 100 µm, and (3) its corrosion resistance, which is unique compared to many other materials. The application of a large thickness is important in cases where fatigue is a problem, for instance, in a situation with point or line contact under high pressure. Fatigue or fatigue wear properties are particularly important when repeated pressure or cyclic push/pull loads are forced upon a surface, causing successive formation of cracks in the surface region, also called surface exhaustion. The result is continuous removal of material from the surface through successive removal of fragments from surface cracks. If the cracked fragments consist of a hard material, this in turn may lead to accelerated abrasive wear.1
Fatigue wear is relatively rare, but it can be observed where a hard brittle coating is coated on top of a ductile material (a material with a large break extension) or a material with a low E-modulus. The phenomenon can be observed on hard anodized surfaces, where the surface is transformed into a brittle ceramic material. Over time, such coatings will crack if the contact area is large compared to the thickness of the actual coating, e.g., a hard chromium layer. Such failures are often misdiagnosed as caused by poor adhesion. The phenomenon can also be observed on nickel plated steel, where the nickel coating has low ductility (brittle like electroless nickel) causing it to fail when exposed to cyclic loads, as described above. Fatigue wear usually occurs in ball bearings, pressure lubricated glide bearings, gearwheel teeth, flanks and fillets (Fig. 3).
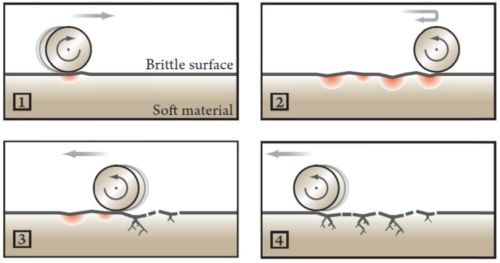
Figure 3 - Fatigue wear caused by a repeated load applied by a roller on a soft base material with a brittle coating. The Hertzian pressure has been exceeded due to deformation of the base material with resulting spalling, cracking and/or pitting.1
Some of the important material properties of hard chromium, which easily can be identified are listed below:
- High hardness.
- Ability to apply large thicknesses greater than 100 µm.
- Ability to handle large components with a weight of a metric ton.
- Low process temperatures compared with other processes such as PVD and thermochemical processes.
- Corrosion resistance comparable to that of stainless steel.
- Usable as a food contact material; unable to introduce allergies as observed with several nickel- or cobalt-containing coatings known from thermal spraying or hardfacing techniques.
- Exhibits easy release properties, as the coating has low surface energy.
- Easy to apply and commonly known worldwide (considerable experience is available - much more than that for other substitutes for hard chromium.
Today hard chromium coatings are used in numerous applications. It was mentioned earlier that removing hard chromium from different technical applications would introduce many new materials science problems and in many cases, it would be difficult to find alternatives, where sufficient experience is available.
One has to accept the fact that, although hard chromium was introduced to the industry as a trial and error coating, it has in many cases become a huge success. The scientific reason for the success and the underlying material properties causing the success are still not fully understood for all applications. That is the reason that replacement in many cases will be guesswork without scientific and technical foundation. There is a lack of experience and knowledge as to what type of coating may substitute for hard chromium in specific applications. If one uncritically replaces hard chromium coatings with presumed alternatives, the result can and will often be catastrophic.
One need only imagine that the hard chromium coatings used on the ring grooves in a piston for large marine diesel engines would suddenly be replaced with a suspected alternative. Such experiments may lead to extensive engine failures with danger for both the crew and ship. Although hard chromium coatings have been on the market for almost 90 years, this case shows that no other coatings have been able to create such security as hard chromium has done. Hard chromium coatings of this type have high durability and typically work in an engine with no problems for more than ten years.
The above postulate does not prove that there is no alternative, but it does show that the hard chromium coating used for ring grooves is difficult to specify from a technical point of view. We only know that hard chromium works. We don´t know why.
Many relevant questions have never been asked or answered. Why does chromium in this specific application not show fatigue, even if it is produced with a high density of cracks? Why is "low surface energy" not a problem in anti-wear and lubrication applications? Why do prolonged high temperature conditions in engines not affect the chromium coating? Particular attention should be paid to the effect of high amounts of impurities in heavy fuels, especially in connection with this type of application. As long as we are not able to answer these kinds of questions, we cannot hope to find a unique performing substitute for hard chromium.
The problem of corrosion can easily be studied by examining the Pourbaix diagram for chromium. The diagram clearly explains why chromium can be used as contact material for food, which is not possible for nickel. The reason is simply that hard chromium passivates in the neutral pH region of the diagram, which coincides with the stability area for water. This is a unique property compared to numerous other materials. Only metals like titanium (Ti) and zirconium (Zr) have similar or better properties.
Figure 4 shows the calculated Pourbaix diagrams for four different metals: Cr, Fe, Ni and Al. A Pourbaix diagram shows the potential E as function of pH, showing the different stable regions for the pure metal, ions and oxides.
E = f(pH)
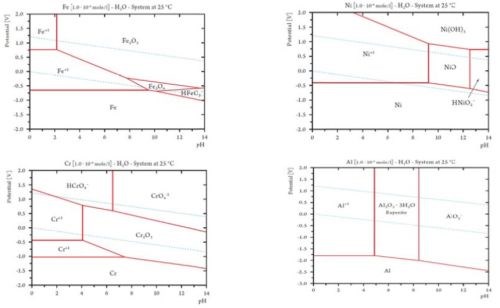
Figure 4 - Pourbaix diagrams calculated for four different metals: Cr, Fe, Ni and Al. A Pourbaix diagram shows the potential as a function of pH, showing the stability regions for the metal, ions and oxides. The stability region for water is situated between the two blue dotted lines. See reference [1] Appendix C for an introduction to Pourbaix diagrams calculated for more metal.
The stability region for water is found between the two dotted blue lines. The lower line describes the reduction of water to hydrogen, whereas the top line describes the oxidation of water to oxygen. Between the two lines, water is stable from a theoretical point of view.
The red lines describe the stability of the selected species (metal ions, metal oxides and pure metal). Observing chromium makes it clear that Cr2O3 is stable in a large pH range and in most of the area where water is stable. Only at a rather high potential - close to the potential for forming oxygen by the oxidation of water - does the passive Cr2O3 start to oxidize to chromate (Cr+6 or CrO4-2).
Cr2O3 + 10OH- ⇔ 2CrO4-2 + 5H2O + 6e-
Under normal conditions, it is very difficult to obtain such a high potential, making the Cr2O3 chromium passivate very stable under normal conditions. It can also be observed that chromium is stable to a pH of about 4. If one examines the Pourbaix diagram for both iron and nickel, it is easy to observe that chromium has a larger passive area where the Cr2O3 coated surface is stable. The Pourbaix diagram for nickel shows that nickel easily releases nickel ions in most of the pH and potential area, which is also the reason why nickel is not accepted as a food contact material. Easily released nickel ions in contact with skin cause nickel-dermatitis. Iron also easily corrodes in the stability area for water, which is not surprising.
Based on the diagrams for iron, chromium and nickel, it is not surprising that the element chromium in stainless steel is responsible for the corrosion resistance. It is also not surprising that the quality of stainless steel is evaluated in terms of chromium content. When the price of nickel increases, it is common for industry to consider using stainless steel with little or no nickel content, i.e., ferritic stainless steel.
Aluminum is often used as food contact material, especially in connection with packaging, and it works quite well under neutral pH conditions. Aluminum forms a very thin but effective passivating layer (Al2O3•3H2O), similar to chromium. When the pH shifts to alkaline or acid regions, aluminum is no longer stable, as shown in the Pourbaix diagram for aluminum (Fig. 4). This statement is in very good agreement with practical observations. It is the main reason why food equipment cannot be manufactured from aluminum, as it starts to corrode when it is treated with alkaline cleaning agents. Such a treatment is necessary to maintain high hygienic standards. It is difficult to remove food contamination such as fat and in several cases eliminate microorganisms, if strong alkaline cleaning agents cannot be used. If we look at the Pourbaix diagram for chromium (Fig. 4), no problem can be identified in the alkaline region. Iron also exhibits stability in the entire alkaline region. These facts explain why stainless steel (an alloy of Cr, Ni and Fe) works quite well in strong alkaline media.
Alternatives to hard chromium
Numerous research groups currently claim to have the solution for replacing hard chromium, but when the argument is treated scientifically, one finds that many important facts and properties have been overlooked. Often high corrosion resistance has to be combined with high hardness and fatigue resistance caused by high Hertzian contact stress. Only a few of these alternate materials have the relevant properties, when combining two or more required specifications, to be a real candidate for replacing hard chromium.
Hardfacing coatings
In order to find such feasible materials, one needs to look in the field of hardfacing for materials where the coating material has high hardness and is stable in a corrosive environment. Often, one ends up with a Stellite®-like material which has been plasma sprayed and further densified through laser fusion.1 Such coatings have already been used with good success in cases where hard chromium coatings are already insufficient. Such processes are generally very expensive because considerable process handling and complex tooling is involved. In general, plasma-sprayed coatings have insufficient mechanical adhesion and exhibit porosity, which can easily lead to corrosion. Therefore laser fusion is an important part of the process.
Nitrocarburizing
It has been claimed that a process called “QPQ” can replace chromium. The process is a specialized type of nitrocarburizing, a case hardening process that increases corrosion resistance by a subsequent oxidation process which forms magnetite (Fe3O4) at the surface. It is sometimes known by the brand names Melonite, Tufftrideor or Tenifer. Three steps are involved in the QPQ process: nitrocarburizing ("Quench"), Polish and post-oxidization ("Quench"). The coating is subsequently treated with hydrophobic agents based on a wax or an oil. The coating will normally pass a salt spray test because water is kept from contact with the surface, preventing the formation of red corrosion products. The coating works well in cases where only abrasive and adhesive wear are the main problems. The coating probably performs better in the case of adhesive wear than does hard chromium. However, with respect to the corrosion resistance of the coating itself, magnetite (Fe3O4) formed by the oxidation step is only stable in a limited pH and E region, according to the Pourbaix diagram shown in Fig. 4. The coating is not stable even at pH 7, if the hydrophobic material is removed. The following chemical reactions can be expected:
4Fe3O4 + O2 + 6H2O ⇒ 12FeO•OH
2FeO•OH ⇒ Fe2O3 + H2O
Physical vapor deposition
Physical vapor deposition (PVD) has been considered as a replacement for hard chromium. It is important here to state that PVD-coating techniques involve very slow coating speeds and the base material typically has to be heated to between 150 and 500°C, depending on the type of coating. Recently, new pulse-based PVD technologies such as HiPMS (High Power Impulse Magnetron Sputtering) show potential in enabling the formation of nitrides such as TiAlN and TiN at lower process temperatures. The PVD process is normally a batch process with limited capacity and limited to components of dimensions below one meter due to the constraints of the vacuum chamber. The thickness of PVD coatings is often below 5 µm. If the coating has any risk of fatigue caused by high Hertzian contact stress, the use of such coatings can be problematic if they are not supported by a hard underlayer hardened through thermochemical processes, plasma nitriding or by the electroplating of a hard coating, fulfilling the mechanical properties according to the Hertzian pressure problem.1
Physical vapor deposition is of interest in connection with the easy-release properties described earlier in this paper, and for hard coatings on top of tool steels or steels which have been case hardened. PVD coatings show many good results and, in particular, CrN, TiAlN, CrTiAlN and diamond-like carbon coatings (DLC) show good and unique properties such as easy release in connection with injection molding, extended lifetime of tools and devices, and low friction for surfaces in relative motion, just to mention a few of the tailored surface properties that can be obtained by applying PVD coatings (Fig. 5). The coatings can be manufactured by reactive magnetron sputtering (DC, MF-pulse, RF and HiPIMS) where a gas (typically nitrogen) simultaneously reacts with the sputtered metal, forming very hard coatings. This type of coating often shows a high corrosion potential similar to that of precious metals, where porosities in thin coatings can be fatal and may cause heavy under-corrosion in service. Among the major drawbacks of PVD coatings are their cost and the fact that the size of the components is limited by the size of the PVD chamber. In addition, the deposition rate is lower than what can typically be obtained by electroplating.

Figure 5 - To the left are seen different machine components PVD-coated with diamond-like carbon, TiN and CrN. To the right is seen a special customized HiPIMS (High Power Impulse Magnetron) PVD unit from CemeCon AG customized for the Danish Technological Institute. (Refer to Reference 1 for further details).
Chromizing
It is also possible to diffuse chromium into steel by a thermochemical process called chromizing, using a pack process. The process forms an iron-chromium coating, which in some cases can be enhanced with carbon, improving hardness, and which has been reported to be even harder than that of hard chromium. Chromizing is a diffusion process where a chromium-containing solid or gas, supplies chromium atoms to the surface of a steel substrate, yielding a chromium concentration between 13 and 30 wt%. The incoming chromium forms chromium compounds with the steel or its alloying elements, resulting in increased hardness in the surface layer. Chromizing by granulation is by far the most widespread process. The process is performed at temperatures above 850°C in an activation gas mixture of hydrogen, H2, and hydrochloric acid, HCl. The chromium source is usually ferrochromium, which reacts with the activation gas according to the following reaction sequence:
Cr + 2HCl ⇒ CrCl2 + H2
Fe + 2HCl ⇒ FeCl2 + H2
The formed CrCl2 will then react with the basis material at the surface:
CrCl2 + Fe ⇒ Cr + FeCl2
CrCl2 + H2 ⇒ Cr + 2HCl
The process conditions need to be adjusted to avoid the formation of chromates. Continuous cleaning of the surface for byproducts such as FeCl2 and HCl is necessary to keep the process active. During the diffusion process, chromium will precipitate as carbides on reaction with carbon, which diffuses from the bulk into the surface layer.
The ternary system consisting of Fe-Cr-C compounds forms complex Fe-Cr-carbides where the chemical composition is determined by the chemical composition of the applied granules, the treatment temperature and the chemical composition of the substrate material. The hardness of the carbide layer formed depends on the carbon content in the substrate material. High hardness is obtained by chromizing the substrate material with a carbon content higher than 0.6 wt%. The hardness of the carbide layer thus formed approaches 1800 HVN. Typical carbide layer thicknesses are in the range of 5-20 μm. Chromium carbide is relatively corrosion resistant with good oxidation resistance, and chromized surfaces can be applied at temperatures up to 600°C. The process is complex and all types of pack processes generate waste, which has to be handled.
Electrochemical processing
If one focuses on electrochemical processes, some interesting candidates can be identified. Nonetheless, they have several drawbacks compared with the original hard chromium process. As early as the 1970s, hardened electroless nickel (EN) was proposed as a potential replacement for hard chromium. Over the years EN has been used in many applications where hard chromium had been used. In the beginning of the EN-era, electroless nickel was also introduced as a dispersion plating process for co-depositing silicon carbide and boron carbide particles. These processes showed very good abrasive wear properties and in some cases performed better than hard chromium. In practice however, dispersion plating is a very complex process, which requires careful rotation and agitation to obtain uniform particle incorporation during the plating process.
If one examines the REACH directive, it might well be concluded that nickel and its alloys will have more restrictions in the future, when used in industry and especially if the coatings will be used in applications having skin contact. It is expected that most nickel plated products will have difficulty in meeting the EU-Nickel Release Regulations, EN 1811:2011.
If one examines the Periodic Table and focuses on the pure metals for electroplating, only two metals remain which probably can be used as wear-resistant coatings (Fig. 6). They are iron (Fe) and manganese (Mn). Cobalt cannot be used because it is already proposed for prioritization for addition into the Annex XIV of the REACH directive.
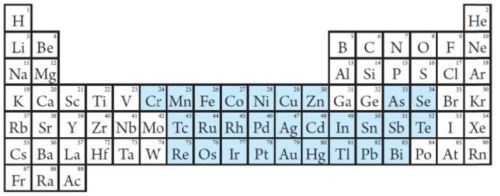
Figure 6 - The blue elements are the pure metals and metalloids that can be deposited electrolytically. Basically only two metals are left which can be used for wear-resistant coatings - iron (Fe) and manganese (Mn).1
The electroplating of iron is not a well-known process. During World War II, iron electroplating was done to conserve nickel and copper. Iron plating nowadays has very limited commercial application and only iron plating for soldering tips and engine cylinders in the automotive industry appear to be known and used on a commercial basis. For many years the electroplating of iron was carried out and even the iron electroforming of phonograph records has been done. Only a few chemical suppliers have really tried to develop a stable electroplating process. There are several reasons to focus on the electroplating of iron. Iron is cheap, harmless and is present in large amounts. Iron can be electroplated as pure iron, as an iron-carbon alloy and as an iron-phosphorus alloy (1-6% wt% P). Depending on the process selected and especially its formulation, iron can be electroplated as a hard coating with a hardness similar to that of hard chromium, but also as a very soft and ductile coating.
Electrodeposited pure iron is relatively corrosion resistant because the corrosion kinetics appear to be very slow. Pure iron is easy to treat with thermochemical diffusion processes and, from a materials science point of view, a number of interesting applications, where electroplating and thermochemical processes can be combined to enable case hardening of the electrodeposited iron layer, will be possible. Such techniques will make it possible to combine steel, which has high strength and cannot be practically case hardened, with a top layer of pure iron, which can be thermochemically treated with a suitable process.
The electroplating of iron-phosphorus also shows some interesting possibilities. The phosphorus can be added as a hyphophosphite salt, yielding hardnesses in the range of 500-1100 HVN with a content of 1-6 wt% P (Fig. 7).
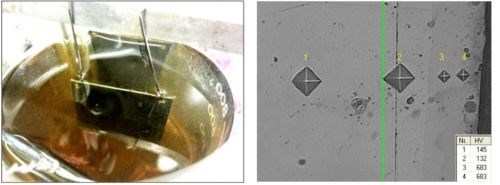
Figure 7 - Left: Test panel with an electroplated iron/phosphorus alloy with 2 wt% P. Right: A cross-section of such a coating with diamond impression showing the hardness results. Such coatings are used for plating pistons and cylinders in the automotive industry.
Some experiments have been made where a duplex layer of electrodeposited iron-phosphorus and pure tin was electrodeposited with the tin layer on top. The coatings were interdiffused in a diffusion alloying process, a thermochemical process, carried out at 225°C for 20 hr. The result was an iron-tin-phosphorus alloy, with an interesting surface morphology for immobilizing dry lubricants. Figure 8 shows the morphology of the coating. In the picture to the left a cross-section is shown and to the right the SEM image shows the surface morphology, consisting of very hard iron-tin intermetallics.
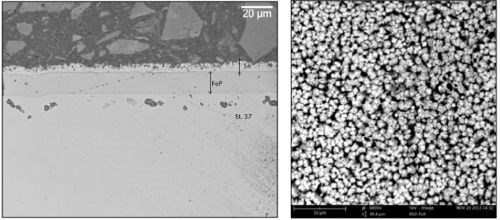
Figure 8 - Duplex layer of electrodeposited iron-phosphorus (2 wt% P) and pure tin diffused into the iron alloy coating.
Preliminary experiments have been carried out measuring the tribological properties for hard chromium, iron-phosphorus and iron-phosphorus with and without dry tin and boron nitride lubricants. Figure 9 compares the different measured friction coefficients. FeP-Sn-BN and FeP-Sn-PE are coatings with FeP-Sn treated with hexagonal boron nitride and PE wax lubricant immobilized in the microstructure. FeP-Sn describes a coating (Fig. 8), where a thin layer of tin is diffused into the FeP coating by a heat treatment of the iron phosphorus alloy coating.
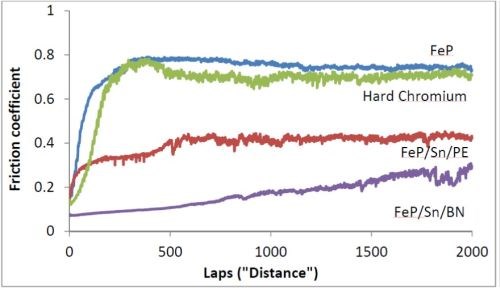
Figure 9 - Friction coefficient for different iron-phosphorus coatings (2 wt% P) compared to hard chromium and coatings of tin diffused into the FeP coating followed by post-treatment with BN and PE-wax.
With respect to the electroplating of iron, the stability of the process is a challenge. The current efficiency depends on the process, but is often in the range 50 – 80%. Ferric (Fe+3) ions will be present in the electrolyte during operation because of air oxidation. The use of insoluble anodes will always lead to an increase in the Fe+3 content of the electrolyte because ferrous iron (Fe+2) is easily oxidized to Fe+3 at the anode. If the Fe+3 concentration is too high, the cathode current efficiency will decrease and the electrodeposited Fe-P layer will become too brittle. In general, an increase in Fe+3 will introduce a high tensile stress in the coating, leading to defects, particularly at corners and edges. A chemical reduction of Fe+3 by oxidation of iron metal is possible according to the following reaction:
2Fe+3 + Fe ⇒ 3Fe+2
This will cause an increase in iron concentration in the electrolyte and the electrolyte will have to be diluted.
Iron plating offers some challenges, particularly in controlling the formation of Fe+3. Currently, new ideas are being tested and soon it may be possible to work with iron baths as easily as with nickel baths. There is no doubt that the process will open up new perspectives and possibilities in the area of materials technology. This will particularly be the case if the iron plating process is combined with thermochemical diffusion processes or diffusion alloying processes where a second metal or element is diffused into the iron coating.
Conclusion
The unique properties of hard chromium coatings were presented and potential alternatives were discussed. A new iron-phosphorus coating process was introduced with deposit hardness values in the range of 500-1100 HVN, depending on the phosphorus content.
Promising tribological results were demonstrated, particularly when the iron-phosphorus coating was combined with a topcoat of electrodeposited tin followed by the formation of a thermal diffusion alloying process involving heating the iron-phosphorus-tin coating to 225°C for 20 hr. Clearly this opens up the development of a totally new family of Fe-P diffusion alloys by combining different alloying elements.
Bibliography
1. P. Møller and L.P. Nielsen, Advanced Surface Technology: A holistic view on the extended and intertwined world of applied surface engineering, ISBN 978-87-92765-23-9 (2013).
2. http://www.athenaallergy.com/Cell-Phone-Dermatitis.html
Related Content
Products Finishing Reveals 2024 Qualifying Top Shops
PF reveals the qualifying shops in its annual Top Shops Benchmarking Survey — a program designed to offer shops insights into their overall performance in the industry.
Read MoreHow to Choose Between Sulfate and Chloride-Based Trivalent Chromium
There are several factors to consider when choosing between sulfate and chloride-based baths for trivalent chromium plating. Mark Schario of Columbia Chemical discusses the differences and what platers should keep in mind when evaluating options.
Read MorePossibilities From Electroplating 3D Printed Plastic Parts
Adding layers of nickel or copper to 3D printed polymer can impart desired properties such as electrical conductivity, EMI shielding, abrasion resistance and improved strength — approaching and even exceeding 3D printed metal, according to RePliForm.
Read MoreA Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.
Read MoreRead Next
Delivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreA ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read More













.jpg;maxWidth=300;quality=90)













