There is a focus and movement across many industrial platforms, especially in the automotive industry sector, to utilize lighter weight materials. Aluminum alloys are an ideal choice for replacing heavyweight steel components, including those traditionally fabricated by steel die-casting processes.
However, aluminum, like all the other metals, becomes susceptible to corrosion (especially pitting corrosion) in the presence of aggressive ions or under specific environmental conditions. Process development work has been completed to optimize conditions for the anodizing of cast aluminum parts with high silicon concentrations. Taking into account the morphology/distribution of the intermetallic species and their impact on the anodic oxide film quality, the results demonstrate how to optimize the entire anodizing process for minimizing their impact, while improving the anodic aluminum oxide layer (AAO) quality.
Metallurgical inspections of the anodized and A356 alloy surfaces were evaluated with optical microscopy (OM); scanning electron microscopy (SEM); and neutral salt spray (NSS) test methods, per ASTMB117/ISO 9227. These methods support verification of the impact from the development results for the optimized process.
The cast aluminum alloy often utilized for replacing steel cast components (for exterior applications) is ISO AlSi7Mg, commonly known as ASTM A356. This is equivalent to SAE 323 or EN AL 42100, which represents a general-purpose, high-strength alloy exhibiting very good casting properties, particularly utilized for fabricating parts subjected to cyclic or vibrational loads.
SILICON TO IMPROVE DIE CASTING
Silicon, an important intermetallic species, is introduced to aluminum as an alloying element for improving the die casting process. This includes the ability to manufacture complex shapes and geometries of parts. However, the presence of high concentrations and gradients of silicon throughout the aluminum alloy (referred to as “rivers of silicon” and shown in the photomicrograph 1a) can create problems in the anodizing process and postanodizing corrosion performance. Alloys with higher silicon concentrations can inhibit the growth of anodic oxide films under galvanostatic and potentiostatic conditions.
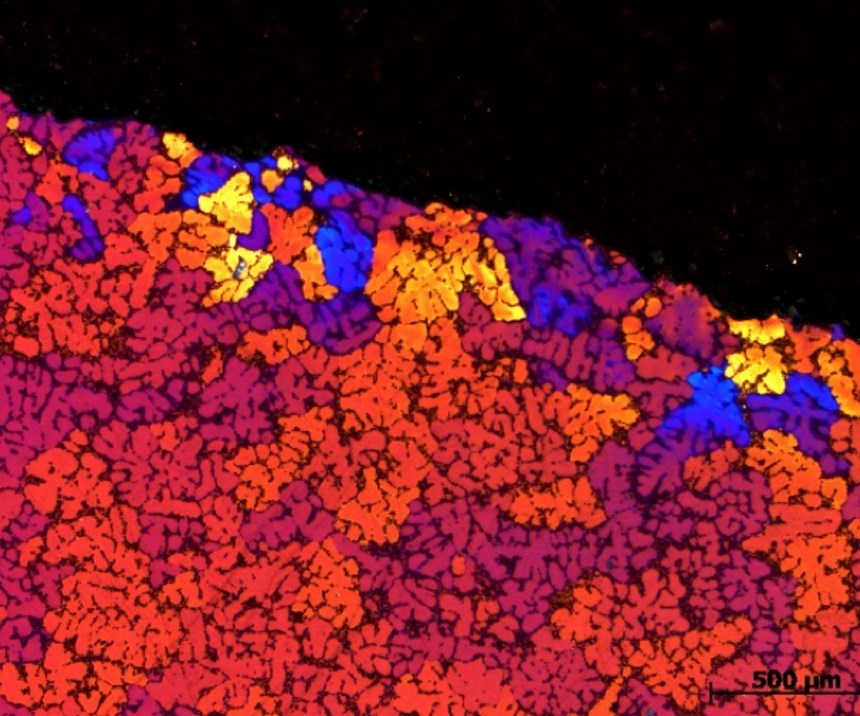
To better investigate and show the effect of the cooling on the surface morphology—and oxide growth—an advanced method is used. The samples are first machined at a 15-degree angle and prepared for optical micrograph so that the topside of the images are actually between the interface of the oxide layer and the metallic substrate. Figure 2 represents a polarized microscopy at 200X magnification of an anodized A356 alloy processed at 15°C for 20 minutes in a sulfuric electrolyte containing 5% oxalic acid and 17 percent sulfuric (GSX electrolyte). The dense small crystals at the top of photomicrograph represent the anodized layer. The aluminum and intermetallic species (including the silicon) are represented in the cross section by the larger grains in the image. As shown, the silicon intermetallic concentrated zones can be seen visually impeding the uniform formation of the anodize oxide layer.
Additionally, these areas of higher concentrations of silicon cause less oxide hardness and negatively affect the postanodizing sealing quality/efficiency. These are important considerations for the overall corrosion performance of the AAO layer. Under the formed anodized oxide film, silicon also acts as galvanic couples that can initiate a pitting phenomenon or cause other types of localized corrosion failures that are detrimental to the use of aluminum for many exterior applications.
Taking into account all these considerations, development work was finalized to optimize the process cycle for anodizing aluminum castings, demonstrating improved success for achieving higher quality performance.
An evaluation of the process optimization was characterized by SEM/EDS and optical microscopy techniques. To validate the results and demonstrate the improvement of the developed process, neutral salt spray tests (NSST) were completed. For these evaluations, samples were directly DC anodized at 59°F in a sulfuric acid-based (170 g/L) electrolyte with different organic additives designed to limit the inhibiting effect of silicon, which causes the anodized layer to be discontinuous. After anodizing the A356 alloy samples, they were colored black with an organic dye at 150°F for 15 minutes. Additionally, they were processed through a cold seal process based on nickel fluoride and pH at 5.9 at 93°F for 15 minutes, followed by a warm water rinse at 150°F. Table 1 provides a detailed overview of the sequences evaluated.
TABLE 1: Process sequence for the Samples A, B, C and D (including the samples dye colored for NSS tests)
|
Sample Codes |
Degreasing |
Special Pretreatment |
Desmut |
Anodizing |
Dye Coloring |
Sealing |
|
A Baseline |
15 minutes |
- |
- |
20 minutes at 59°F with 179 g/l H2SO4 |
At 150°F |
Cold Seal 15 minutes |
|
Warm Rinse |
||||||
|
B |
15 minutes |
- |
- |
20 minutes at 59°F with 179 g/L H2SO4 and 30 g/L Organic Additive |
At 150°F |
Cold Seal |
|
Warm Rinse at 150°F for |
||||||
|
C |
15 minutes |
4 minutes at 195°F |
2 mins at 95 F |
20 minutes at 59°F with 179 g/L H2SO4 and 30 g/L Organic Additive |
At 150°F |
Cold Seal |
|
Warm Rinse at 150°F for |
||||||
|
D |
15 minutes |
10 minutes at 195°F Acidic Based |
2 mins at 95 F |
20 minutes at 59°F with 179 g/L H2SO4 and 30 g/L Organic Additive |
At 150°F |
Cold Seal |
|
Warm Rinse |
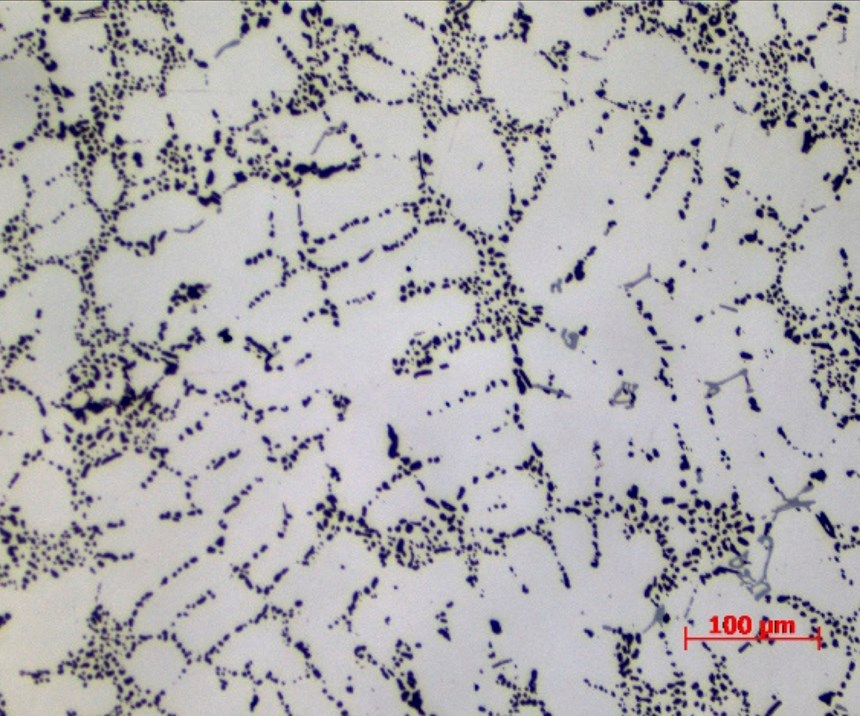
To overcome problems created by too high concentrations of silicon in the casting alloy and to obtain a greater oxide density and a harder, uniform thickness in the AAO layer, a new acid immersion pretreatment process is developed to reduce silicon intermetallic heavy zones from the part surfaces. The specially formulated proprietary acid-based process was applied to the cast parts for 10 minutes at 90°C, resulting in the silicon being more evenly distributed over the aluminum surface.
This surface condition after the pretreatment is much more suitable for the resulting anodizing of the aluminum, as shown in Figures 3a and 3b. This increase in uniformity of the silicon distribution is a result of the decrease in their size and shape that allows the anodizing process to continue more homogeneously and at a faster formation rate. The overall result is a 30 to 40% increase in oxide layer thickness compared to the standard reference under similar operating conditions. Such a dense and homogenous AAO layer result has higher corrosion performance and greater resilience against the fading of the dye-colored samples.
OPTIMIZING THE ANODIZING CHEMICAL ELECTROLYTE
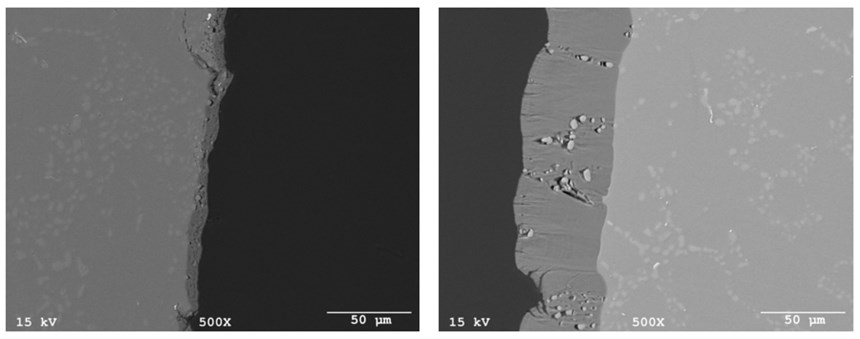
Further improving the AAO layer properties, the next step was optimizing the anodizing chemistry electrolyte. Unlike oxalic acid additives to sulfuric acid electrolytes, there are developments of new organic-based additives available for the anodizing electrolyte that function to overcome the negative effect of the intermetallics of silicon on the AAO characteristics. These additives help to control the dissolution of aluminum and function to incorporate the silicon into the oxide layer with minimal detrimental impact on the coating layer quality. Their use increases the electrolyte conductivity; and the heat generated during anodize layer formation can be more efficiently removed from the metal-electrolyte interface, resulting in increasing efficiency of the oxidation reactions.
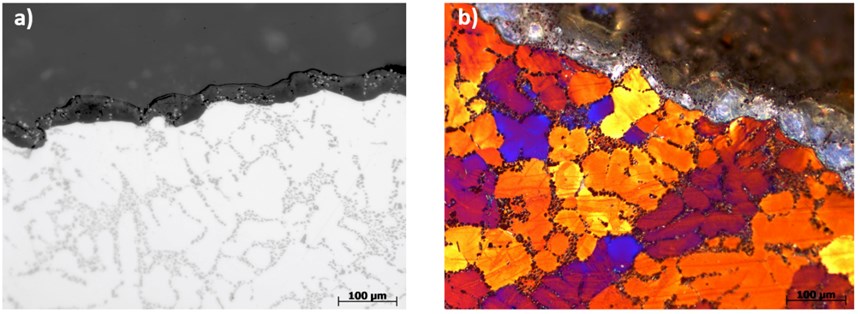
By incorporating the use of a proprietary organic additive blend in the anodizing electrolyte, the negative effects of the silicon intermetallic species are suppressed. The resulting AAO layer has improved density and a thicker oxide film (as shown in Figure 4b) in comparison to the standard GSX anodizing (in Figure 4a referenced in Figure 2).
Combined with the new immersion pretreatment process, the incorporation of the anodizing organic additive (Sample D) additionally increased the oxide layer thickness about four times under the same operating parameters for the GSX electrolyte (sample A). Next, the overall performance and success of the improved sequence (using the special pretreatment and organic additive package to the anodizing electrolyte for creating the optimum oxide layer) was easily evaluated through laboratory NSST testing.
The processes in Table 1 were carried out for four sequences, which are referenced in Figure 5. The samples A, B, C and D were processed accordingly to demonstrate the improvements on the corrosion performance and fade resistance of the anodized A356 cast aluminum.
The additional processing of these anodized surfaces (through an organic black dye system) was completed for samples B, C and D to help visually see more clearly the early formation of corrosion spots on the aluminum. Additionally, the fade resistance of the surface is also a gauge of the AAO layer performance with respect to corrosion resistance.
All were evaluated after 800 hours of NSST exposure. As shown in the baseline sample A, there is base metal corrosion and some color change observed. In agreement with SEM images, the best corrosion behavior was achieved for samples C and D with the incorporation of the special pretreatment and use of organic additive in the anodizing electrolyte.
For sample B, demonstrating the impact of the organic additive on the anodizing solution does not show any corrosion, but the presence of the surface color change indicates the importance of oxide film thickness on color integrity.
In many applications for anodizing cast aluminum alloys, color retention and stability (while being exposed to many environmental factors) is just as critical as resistance to corrosion. By incorporating the use of these organic additive types in the sulfuric anodizing process, and utilizing the special pretreatment process, the homogeneity and the thickness of the oxide layer can be improved for cast aluminum alloys with higher silicon content. This also provides improved corrosion resistance and the ability to provide a greater range of color possibilities that have greater resilience against fading of the dyed surface over time while in service.
This paper is a summary of work carried out by Coventya’s Can Akyil, Güney Akdas, Pınar Afsin, Mesut Akkaya, Mustafa Urgen, Istanbul Technical University, Department of Metallurgical and Materials Engineering, 34469, Maslak, Istanbul –Turkey and Politeknik-Coventya Turkey.
Related Content
Trivalent Chrome Overview
As the finishing industry begins to move away from the use of hexavalent chromium to trivalent chromium, what factors should finishers consider as they make new investments? Mark Schario, chief technology officer for Columbia Chemical offers a helpful overview of this complicated topic.
Read MoreHow to Maximize Nickel Plating Performance
The advantages of boric acid-free nickel plating include allowing manufacturers who utilize nickel plating to keep up the ever-changing regulatory policies and support sustainability efforts.
Read MoreHow to Choose Between Sulfate and Chloride-Based Trivalent Chromium
There are several factors to consider when choosing between sulfate and chloride-based baths for trivalent chromium plating. Mark Schario of Columbia Chemical discusses the differences and what platers should keep in mind when evaluating options.
Read MoreSuccessful South African Plater Beating the Odds
Remaining focused on quality and reliability, Team Plating Works stays profitable in a volatile and challenging economy.
Read MoreRead Next
A ‘Clean’ Agenda Offers Unique Presentations in Chicago
The 2024 Parts Cleaning Conference, co-located with the International Manufacturing Technology Show, includes presentations by several speakers who are new to the conference and topics that have not been covered in past editions of this event.
Read MoreEpisode 45: An Interview with Chandler Mancuso, MacDermid Envio Solutions
Chandler Mancuso, technical director with MacDermid Envio discusses updating your wastewater treatment system and implementing materials recycling solutions to increase efficiencies, control costs and reduce environmental impact.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More










.jpg;maxWidth=300;quality=90)












